-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2012; 2(4): 67-72
doi: 10.5923/j.ajps.20120204.04
Effect of Reaction Conditions on the Uptake of Selected Heavy Metals from Aqueous Media using Composite from Renewable Materials
L. O. Ekebafe 1, M. O. Ekebafe 2, G.O. Erhuaga 1, F.M. Oboigba 3
1Department of Polymer Technology, Auchi Polytechnic, P.M.B 13, Auchi, Edo State, Nigeria
2Chemistry Division, Nigerian Institute for Oil Palm Research, P.M.B 1030, Benin City, Edo State
3Department of Chemistry, University of Benin, Benin City, Nigeria
Correspondence to: L. O. Ekebafe , Department of Polymer Technology, Auchi Polytechnic, P.M.B 13, Auchi, Edo State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study is aimed at developing a value added biosorption product and a cost effective biotechnology for the uptake of heavy metals and organic compounds from aqueous media. The practical problems of cellulose solubility at low pH aqueous systems, gel forming behavior and mass transfer limitations were overcome in this study by coating bamboo culms cellulosic on bamboo culms carbon to form a rigid matrix structure of better mechanical strength, the coating process yielded a stable granular composite adsorbent that was stable under acidic conditions. The adsorption capacity was evaluated by measuring the extent of adsorption of chromium, cadmium, and lead metal ions, from aqueous media under equilibrium and reaction conditions. Equilibrium data at optimized condition yielded the following uptake efficiency values: 93% Cr, 76%Pb, and 82% Cd. The composite compared with other adsorbents also prepared showed better performance.
Keywords: Cellulosic, Biosorbent Composite, Heavy Metals Adsorption, Bamboo Culms
Article Outline
1. Introduction
- Environmental studies have revealed widespread contamination of water by different chemicals used in the chemical industry during manufacturing process[1]. These chemicals include organic compounds, heavy metals and other pigments like dyes in the textile industry. At least 20 metals are classified as toxic and half of these are emitted into the environment in quantities that pose risks to human health[2]. Most of these chemicals, which are toxic to human health and aquatic lives cannot be recycled and are discharged into the environment via streams, rivers, lakes. Some of these constitute the major source of contaminants in portable water supply to humans and livestock. These chemicals find their way into ground water, which cause pollution and could impair plant growth and result to a great risk to human health and the environment[2,3].Efficient removal of these pollutants from the environment is still a problem. Over the years attempts have been made to provide decolourizing type carbon from raw materials like coal, groundnut husks, coconut shells, palm kernel shell, plantain peel, walnut shells, oil palm empty fruit bunches, Bamboo culms, maize cobs, cocoa pods, saw dusts etc[4-8].A wide range of physical and chemical processes is available for the uptake of heavy metals and organics from aqueous media, such as electro-chemical precipitation, ultrafiltration, ion exchange and reverse osmosis[6-8]. The problem associating with the use of precipitation is sludge production. Ion exchange, being a better alternative method is not economically appealing because of high operational cost. Adsorption using commercial activated carbon(CAC) from petroleum based materials can remove heavy metals and organics from aqueous media, However, CAC remains an expensive material for heavy metal uptake, which has caused interest to be shifted to the use of other low cost and readily available agricultural products as precursor for the preparation of activated carbon.Natural biopolymers from renewable sources because of their capability to lower heavy metal-ion concentration to as low as parts per billion have become materials of attraction. Among the many other low cost sorbents identified, cellulosic from agricultural waste has high sorption capacity for several metal ions[9].Bamboo is a naturally occurring composite material which grows abundantly in most of the tropical countries. It is considered a composite material because it consists of cellulose fibers imbedded in lignin matrix. Cellulose fibers are aligned along the length of the bamboo providing maximum tensile flexural strength and rigidity in that direction[4].Since bamboo species are invasive and spread very fast, uncared bamboo species also cause environmental problems. Increased research during the recent years has considerably contributed to the understanding of bamboo as well as to improved processing technologies for broader uses.The active binding sites of cellulose are not readily available for sorption and poses problems for developing commercial applications. Transport of the metal contaminants and organics to the binding sites plays a very important role in process design. Therefore, it is necessary to provide physical support and increase the accessibility of the metal binding sites for process applications.In this communication, an attempt was made to overcome these mass transfer limitations by synthesizing a biosorbent composite by coating cellulose on the surface of bamboo culm carbon and evaluating its equilibrium adsorption properties. The combination of the useful properties of bamboo carbon and that of natural cellulose, could introduce a composite matrix with many application and superior adsorption capabilities. Using synthetic contaminated water, the heavy metals uptake by bamboo carbon coated with cellulose and acid treated bamboo carbon adsorbents were compared.
2. Materials and Methods
2.1. Materials
- Bamboo culms were obtained from Igieduma village, along Benin-Auchi Expressway, Edo State, Nigeria. All the other reagents used were of analytical grade, and used as received. Commercial activated carbon was obtained from the Port Harcourt Refinery and Petrochemical Company, Port Harcourt, Nigeria.
2.2. Preparation of the Bamboo Culms Carbon (BCC) and Oxidizing the Carbon with Nitric Acid
- Matured bamboo culms were harvested and cut into small strips with saw blade. Samples of 1kg each were weighed and heated to 500℃ for three hours using the METM-525 Muffle furnace. The carbonized culms were then milled to fine powder, and sieved through a mesh size of 150µm. The carbon particles that passed through the screen were collected, characterized and designated as Bamboo culms carbon (BCC).Portion of the carbon prepared from bamboo culms were then treated with 2% HNO3(v/v) in an incubator at 110℃ for 24h and soaked with deionized water until the solution pH was stable. Then the adsorbent was soaked in 2%NaHCO3(w/v) till any residual acid left was removed. Finally, the sample(hereafter referred to as acid treated bamboo culms carbon-ABCC) were dried overnight in an oven at 110℃, cooled at room temperature, and stored in a dessicator until further use[10]
2.3. Preparation of Bamboo Culms Cellulosic
- Matured bamboo culms harvested and cut into small strips with saw blade were grounded in the mill. The material was then placed in a shaker with sieves to pass through a 425-μm mesh sieve yet retained on a 250-μm mesh sieve. The resulting material was placed in glass jars labeled with appropriate designation for the preparation.The delignified cellulosic was isolated using the method described by Brendel O. et al[11].50 g of cellulosic was slowly added to 1000 ml of 10 wt% oxalic acid with constant stirring. The mixture was also heated to 45℃ to facilitate mixing. At room temperature, the cellulosic-oxalic acid mixture formed a whitish viscous gel[12].
2.4. Surface Coating of ABCC and BCC with Cellulosic
- About 500 ml each, of the cellulosic was diluted with water and heated to 45℃. About 500 g each of the ABCC and BCC were slowly added to the diluted cellulosic and mechanically agitated using a rotary shaker at 150 rpm for 24 hrs. The cellulosic coated ABCC and BCC were then washed with deionized water and dried. The process was repeated three times to form a thick coating of cellulosic on the ABCC and BCC surfaces. The cellulosic coated ABCC and BCC (now referred to as cellulosic coated acid treated composites, CCAC and Cellulosic coated composites, CCC, respectively) were removed and neutralized by putting them in 0.5% NaOH solution for 3 hrs. The CCAC and CCC were then extensively rinsed with deionized water and dried[13].
2.5. Characterization of the BCC
- The pH was determined by immersing 1.0g samples in 100ml of deionised water and stirring for 1h. Bulk density was determined by a tamping procedure described by Ahmenda et al[14]. The resistance of the active carbon to mechanical abrasion was determined by measuring the percent solids retained after the carbon was stirred in acetate buffer(pH 4.7) at 250rpm for 2h[15].Surface area of the carbon was determined by the iodine adsorption method[16]. The amount of iodine adsorbed from aqueous solution was estimated by titrating a blank with standard thiosulphate solution and compared with titration against iodine solution containing the sample.
2.6. Equilibrium Uptake Studies of Metal Ions
- Equilibrium uptake experiments were carried out using CCC, CACC, and CAC adsorbents. Potassium dichromate(K2Cr2O7), Cadmium sulphate(CdSO4), and Lead nitrate(Pb(NO3)2 were used as the source of Cr(VI), Cd(II), and Pb(II) respectively in the synthetic aqueous media. The Cr, Cd, and Pb stock solutions were prepared by dissolving 0.745 g of K2Cr2O7; 0.521g of CdSO4; 0.828g of Pb(NO3)2 in 250 ml of deionized water respectively.Adsorption experiments were carried out at ambient temperature using the optimum conditions of all pertinent factors, such as pH, dose agitation speed, and contact time[17]. Subsequent adsorption experiments were carried out with only optimized parameters.The change in concentration of the heavy metal ions due to adsorption was determined using the atomic absorption spectrophotometer(AAS).The adsorption efficiency(E) of adsorbent was defined as:E(%)=[(Co- C1)/ Co] x 100Where; Co and C1 are the initial and equilibrium concentration of metal ions solution(mg/ l), respectively.To ensure the accuracy, reliability, and reproducibility of the collected data, all the experiments were carried out in triplicate and the mean values of three data sets are presented. The results are as presented in Table 2-6.
3. Results and Discussion
3.1. Bamboo Culms Carbon
- Bamboo culms have been used to produce quality activated carbon, as other biomass, because of their inherent high densities and carbon content[18, 19]. In this study the carbon from the bamboo culms was prepared according to the method described by Ishak and Baker[20].Some of the characteristics of the carbon obtained from bamboo culms are as presented in Table 1.
3.2. Effect of Reaction Conditions on the Adsorption of the Metal Ions
- The influence of operational parameters such as amount of adsorbent, agitation speed, initial pH and contact time were investigated. The results were expressed as the removal efficiency(E) of the adsorbent on metal ions, which was defined asE(%)=[(Co- C1)/ Co] x 100 , where Co and C1 are the initial and equilibrium concentration of metal ions solution(mg/l), respectively. The metals ion concentration was determined using AAS.
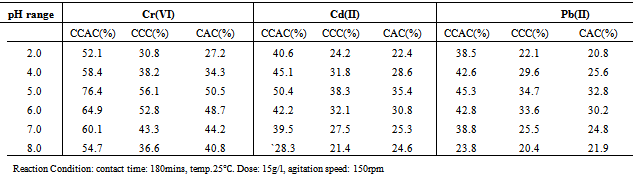
3.2.1. Effect of pH
- pH is an important parameter for adsorption of metal ions from aqueous solution because it affects the solubility of the metal ions, concentration of the counter ions on the functional groups of the adsorbent and the degree of ionization of the adsorbate during reaction[12].The effect of pH on the metal ions removal efficiency was examined by using varied pH from 2.0 to 8.0. As shown in Table 2, the uptake of free ionic metal ions depends on pH, where optimal metal removal efficiency occurs at pH 5 and then decreasing at higher pH. Removal efficiency for CCAC increased from 38.5% to 76.4% over pH range from 2.0 to 8.0.The CCC and CAC adsorbents, also showed similar trends but with much lower removal efficiency and slight different optimum pH value. The adsorption efficiency is as result of the fact that the pH values for these materials are at a lower pH range as the adsorbent, unlike values reported for most chemically untreated commercial activated carbon[22]. It can be mentioned that oxidization of bamboo culms carbon with nitric acid yielded acidic surface due to the introduction of oxygen-containing functional group[23].The oxidative treatment of ABCC with nitric acid introduces more acidic C=O groups on the surface of ABCC[16,24]. The electrostatic interaction between cellulose and the more negatively charged ABCC is improved and this prevents any tendency of cellulose to agglomerate. Increase in the availability of active binding sites on the cellulose for adsorption of the metal ions at low pH conditions is enhanced. It has also been suggested that formation of more acidic surface oxides on the carbon surface enhances its hydrophilic character and hence improve the hydrodynamic flow[25].On the other hand, cellulose coated beads, showed lower adsorption capacity, probably due to less efficient coating resulting in lesser acidic surface oxides. The interaction may not be very strong and the cellulose may agglomerate to a certain degree and become more soluble at low pH and hence reduces the availability of active binding sites on the cellulose for adsorption.Similar bioreduction process can also be accomplished using alfalfa, seaweed, and some lyophilized plant tissue [26, 27].
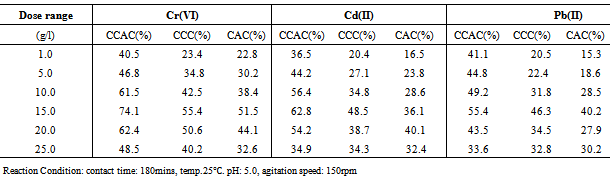
3.2.2. Effect of Dose
- The influence of metal ions sorption on amount of adsorbent was studied by varying the amount of adsorbents from 1.0 to 25 g/l, while keeping other parameters(pH, agitation speed, and contact time) constant.
|
|
|
|
|
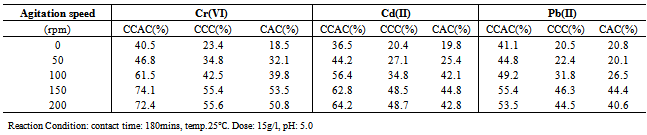
3.2.3. Effect of agitation speed
- The effect of agitation speed on removal efficiency of metal ions was studied by varying the speed of agitation from 0(without shaking) to 200 rpm, while keeping the other factors constant.As observed from Table 4, the metal ions removal efficiency generally increased with increasing agitation speed. The metal ions removal efficiency of CCAC adsorbent increased from 61.5% to 74.1% when agitation speed increased from 100 rpm to 150 rpm and the adsorption capacity appears relatively constant for agitation rates greater than 150 rpm. These result can be associated to the fact that the increase of the agitation speed, improves the diffusion of metal ions towards the surface of the adsorbents. This also indicates that a shaking rate in the range 100-200 rpm is sufficient to ensure that all the surface binding sites are made readily available for metal uptake.
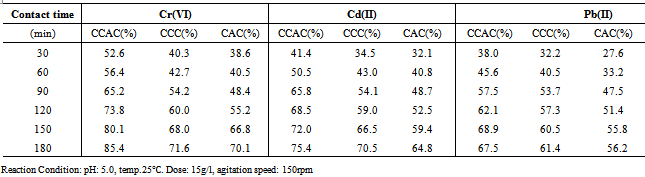
3.2.4. Effect of contact time
- Result in Table 5 indicates that the metal ions removal efficiency increased with an increasing contact time before equilibrium is reached. Other parameters were kept optimum, while temperature was kept at 25℃.It is observed that metal ions removal efficiency of CCAC increased from 52.6% to 85.4% when contact time was increased from 30 to 180 min. Optimum contact time for CCAC, CCC and CAC adsorbents was found to be 180 min. Greater availability of hydroxyl functional group on the surface of cellulose, which is required for interaction, significantly improved the binding capacity and the process proceeded rapidly. This result is important, as equilibrium time is one of the important parameters for an economical wastewater treatment system.
3.3. Metal Ions Uptake from Aqueous Medium at Optimized Condition
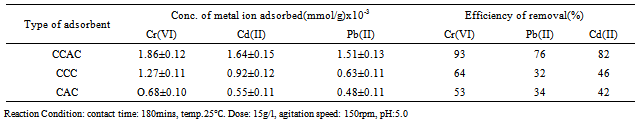
- Structural aspects of the polymeric backbone are important factors affecting metal ions sorption. The prepared biosorbent composite removes metal ions both by adsorption on hydroxyl group and also by sorption in the bulk of composite. Therefore, the structure of a polymeric composite affects the level of polymer interaction with the media and the provision of active sites to adsorb or coordinate metal ions. Hence the sorption behaviour and the quantity of metal ions taken up depend; in addition to the attributes of structural aspects of the polymeric backbone but also on the functional groups(active binding sites), and the nature of the material, which are important factors affecting metal ions sorption. It is clear from Table 6, that the metal ions uptake and removal efficiency of Cr were higher than Cd, and Pb. This can be attributed to the fact that the Cr ion has a lower atomic radius than other metal ions and consequently its adsorption by the composite is high. In general, the amount of metal ions uptake by ion exchanger is affected by the electronegativity and hydrated values of metal ions. The sequence of metal ions sorption were as follow: Cr >Cd >PbThe coating of the bamboo culms carbon affected the metal ions uptake and removal efficiency by opening up the binding sites in the composite network and activating hydroxyl group into more active binding sites. From table 6, it is seen that the metal ions uptake and removal efficiency of CCAC was higher than that of CCC and CAC.
4. Conclusions
- It has been shown that the use of biosorbent composite from renewable materials for heavy metal ions uptake is technically feasible, eco-friendly, low cost and with high efficiency. The oxidative treatment of the bamboo carbon with nitric acid introduces more acidic C=O groups on the surface of carbon. This enhanced the electrostatic interaction between cellulose and the more charged carbon and this prevents any tendency of cellulose to agglomerate. This helped to increase the availability of active binding sites on the cellulose for adsorption of Cr, Cd, and Pb at acidic conditions. Besides that, being composed entirely of agricultural waste, it helps in reduction of waste generation and added value to the waste. The adsorbent can be regenerated by using 0.1M sodium hydroxide, and therefore can be reused. This adsorbent can be a good candidate for adsorption not only for these heavy metals selected; but also others in industrial and municipal wastewater stream.
References
| [1] | S.O. Ajayi, S.A,Adelaye(1977): Pollution studies of Nigerian Rivers; Bulletin of the C.S.N Vol 2, 72-78 |
| [2] | A. Kortenkamp; M. Casadevall; S.P. Faux; A. Jenner,; R.O.J. Shayer; N. Woodbridge,and P. O’brien,(1996): A role for molecular oxygen in the formation of DNA damage during the reduction of the carcinogen chromium(VI) by glutathione, Archives of Biochemistry and Biophysics, vol. 329, no. 2, p. 199-208. |
| [3] | J.F. Blais, S. Dufresne and G. Mericer(2000): State of art of technologies for metal removal from industrial effluents. Rev. Sci.Envir. 12(4): 687-711. |
| [4] | E.C. Bernardo, R. Eqashira, and J. Kawasako(1997): Decolourisation of molasses wastewater using activated carbon prepared from cane bagasse. Carbon 35(9); 1217-1221. |
| [5] | H.P. Boehnm(1994): Some aspects of surface chemistry of carbon blacks and other carbons. Bioresource Technol; 32:759-770. |
| [6] | S. Rengaraj; K.H. Yeon, and S.H. Moon,(2001) Removal of chromium from water and wastewater by ion exchange resins. Journal of Hazardous Materials,vol. 87, no. 1-3, p. 273-287. |
| [7] | L. Yurlova.; A.Kryvoruchko, and B. Kornilovich,(2002) Removal of Ni(II) ions from wastewater by micellar-enhanced ultrafiltration. Desalination, vol. 144, no. 255-260. |
| [8] | Y. Benito, and M.L. Ruiz,(2002) Reverse osmosis applied to metal finishing wastewater. Desalination, vol. 142, no. 3, p. 229-234. |
| [9] | M.V. Deshpande,(1986): Enzymatic degradation of chitin and its biological applications. Journal of Scientific and Industrial Research, Sci. Ind. Res., vol. 45, p. 277-281. |
| [10] | J.W. Shim; S.J. Park, and S.K. Ryu,(2001) Effect of modification with HNO3 and NaOH by pitch-based activated carbon fibers. Carbon, vol. 39, no. 11, p. 1635-1642. |
| [11] | O. Brendel; P.P.M Iannetta, and D. Stewart,(2000): A rapid and simple method to isolate pure-cellulose. Phytochemical Analysis, 11: 7–10. |
| [12] | Salfuddin Nomanbhay and Kumaran Palanisamy(2005): Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal, Elect. Jour. Of biotech.vol 8 No. 1pp. 43-53. |
| [13] | S. Babel, and T.A. Kurniawan,(2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agent and/or chitosan. Chemosphere, vol. 54, no.7, p. 951-967. |
| [14] | M. Ahmedna, M. Johnson, S.J. Clarke, W.E. Marshal and R.M. Rao(1997): Potential of agricultural by-product based activated carbon for use in raw sugar decolorisation. J.Sci.Food Agric. 75: 117-124. |
| [15] | M.M. Johns W.E. Marshal. and C.A.Toles(1999): The effect of activation method on the properties of pecan shell activated carbon. J.chem..Technol Biotechnol; 1037-1044 |
| [16] | A. Toles E. Marshal M. Johns H.. Wantell A. Mealoon(2000): Acid activated carbons from almond shells, physical, chemical and adsorptive properties and estimated cost of product. Bioresource Technol 71; 87-92. |
| [17] | S. Chakravarty,; V. Dureja; G. Bhattacharyya; S. Maity, and S. Bhattacharjee,(2002) : Removal of arsenic from groundwater using low cost ferruginous manganese ore. Water Research; no. 36, vol. 3, p. 625-632. |
| [18] | M. Normah.; K.C. Teo and A.P. Watkinson. Preparation and characterization of activated carbon derived from oil palm shells using a fixed bed pyrolyser. In:M.A.Hashim, ed. Bioproducts Processing: Technologiesfor the tropics. Institute of Chemical Engineers, Rugby,United Kingdom, 1995, p. 93. ISBN |
| [19] | J. Guo and A.C. LuaU Preparation and characterization of adsorbents from oil palm fruit solid waste. Journal of Oil Palm Research Malaysian Palm Oil Board, 2000, vol. 12,p. 64-70. |
| [20] | Z.A.M. Ishak; A.A. Bakar,(1995): An investigation on the potential of rice husk ash as fillers for epoxidized natural rubber, Eur. Polym. J. 31(3), 259 – 269 |
| [21] | T. Gotoh.; K. Matsushima and K.I. KikuchiI, .Preparation of alginate-chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere, April 2004, vol. 55, no. 1, p. 135-140. |
| [22] | J.A. Menendez; J. Phillips; B. Xia, and L.R. Radovic,(1996): On the modification and characterization of chemical surface properties of activated carbon: in the search of carbons with stable basic properties. Langmuir, vol. 12, no. 18, p. 4404–4410. |
| [23] | P.C.C.Faria; J.J.M. Orfao, and M.F.R.Pereira,(2004): Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Research, vol. 38, no. 8, p. 2043-2052 |
| [24] | P.R. Wittbrodt and C.D. Palmer, Effect of temperature, ionic strength, background electrolytes and Fe (III) on the reduction of hexavalent chromium by soil humic substances. Environmental Science and Technology, 1996, vol. 30, no. 8, p. 2470-2477. |
| [25] | C.M. Lytle; F.W. Lytle; N. Yang; J.H. Qian; D.Hansen; A. Zayed, A. and N. Terry. Reduction of Cr (VI) to Cr (III) by wetland plants: Potential for in situ heavy metal detoxification. Environmental Science and Technology, 1998, vol. 32, p. 3087-3093. |
| [26] | M.R. Mostafa, Adsorption of mercury, lead and cadmium ions on modified activated carbon. Adsorption Science and Technology, 1997, vol. 15, no. 8, p. 551-557. |
| [27] | T.J. Olin; J.M. Rosado; S.E.Bailey and R.M. Bricka, Low cost sorbents screening and engineering analysis of zeolite for treatment of metals contaminated water and soil extracts – final report. Report SERDP, 1996; 96-387, prepared for USEPA and SERDP. |
| [28] | Y. Leon C.A. Leon; J.M. Solar.; V. Calemma and L.R. Radovic. Evidence for the protonation of basal plane sites on carbon. Carbon, 1992, vol. 30, no. 5, p. 797-811. |
| [29] | L.R. Radovic; I.F. Silva; J.I. Ume; J.A. Menendez.; Y. Leon C.A. Leon and A.W. Scaroni An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electrondonating functional groups by chemically modified activated carbons. Carbon, 1997, vol. 35, no. 9, p. 1339-1348. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML