-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2012; 2(4): 62-66
doi: 10.5923/j.ajps.20120204.03
Copolymerization of 2,2-Diallyl-1,1,3,3-tetraethylquanidinium Chloride with N-(4-Acetylphenyl)maleimide
Marina N. Gorbunova
Institute of Technical Chemistry Ural Branch of Russian Academy of Sciences, Perm, 614013, Russia
Correspondence to: Marina N. Gorbunova , Institute of Technical Chemistry Ural Branch of Russian Academy of Sciences, Perm, 614013, Russia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The free-radical copolymerization of 2,2-diallyl-1,1,3,3-tetraethylguanidinium chloride with N-(4- acetylphenyl)maleimide is studied in bulk and in organic solvents. It is shown that copolymerization in bulk and in solution proceeds to form copolymers with random distribution of monomer units. The kinetic laws of the reaction are investigated, and the relative activities of the monomers are determined. It is found that 2,2-diallyl-1,1,3,3-tetraethylguanidinium chloride is involved in copolymerization with N-substituted maleimides to give rise to pyrrolidinium structures.
Keywords: Radical Copolymerization, Quanidinium Salt, N-(4-Acetylphenyl)Maleimide, Kinetics
Article Outline
1. Introduction
- Maleimides have found wide use as thermoreactive binders in engineering, electrical insulation, and technical items because materials formed on their basis are resistant to radiation and possess high fire resistance; moreover, their dielectric properties are stable up to 200–250℃[1]. It is known that N-substituted maleimides homopolymerize according to the radical mechanism[2,3] and are involved in copolymerization with styrene[4–9], butadiene[4], (meth) acrylic acid and its esters[4,11], vinyl ethers[10–12], and vinylketones[13]. In copolymerization with electron-donor monomers, they, being electron acceptors, form copolymers with a high tendency of monomer units toward alternation[4,9,11,12].Compounds containing quanidine groups show a broad spectrum of bactericide effects, and they are used as medicines and fungicides[14–16]. That is why quanidine group introduction into high molecular-weight compounds is undoubtedly of interest.2,2-Diallyl-1,1,3,3-tetraethylguanidinium chloride(AGC) shows promise for this purpose.The copolymerization of this monomer with N-phenyl- and N-p-carboxyphenylmaleimide was reported in[17]. It was shown that copolymerization proceeds to form copolymers with a high tendency toward alternation of monomer units.No data is available on the copolymerization of AGC with N-(4-acetylphenyl)maleimide; however, the synthesis and characterization of copolymers based on AGC is of great interest since the mentioned compounds can be used in medicine and biotechnology.In the present study, the copolymerization of AGC with N-(4-acetylphenyl)maleimide (APMI) was studied.
2. Experimental
2.1. Materials
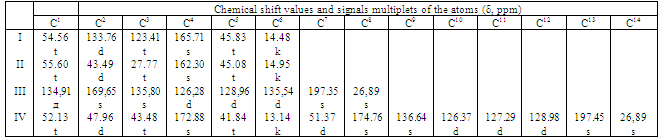
- 2,2-Diallyl-1,1,3,3-tetraethylguanidinium chloride was synthesized as described in[18]. Tetraethylurea (1 mol) was dissolved in dry benzene (2.5 mol). Phosgene was bubbled through the solution at 9-15℃ at the intensive stirring until the reaction was completed (control by gas-liquid chromatography). Then the reactive mixture was heated slowly and was boiled until the gas stopped emanating. It was followed by the cooling of the reactive mixture and dry diallylamine (2.4 mol) was added dropwise with the constant intensive stirring. Then the reactive mixture was stirred for two hours at 50-60℃ and sodium hydroxide (1.2 mol of 50% aqueous solution) was added dropwise to the mixture. After that the reaction mixture was filtered, the filtrate was evaporated under vacuum at 70℃. The obtained AGC was thoroughly rinsed with dry acetone to remove the residual NaCl. Finally, acetone was removed by distillation. The yield of AGC was 70 % from the theory. The purity of AGC was determined via elemental analysis and 13CNMR spectroscopy. According to elemental analysis, the contents of elements are as follows: C, 62.42(calcd., 62.61); H, 10.67(calcd., 10.43); N, 14.58% (calcd., 14.61); and Cl, 12.32% (calcd., 12.35). The chemical shifts (δ, ppm) and multiplicity of the 13CNMR spectrum of AGC are given in Table 1.APMI was synthesized as described in[19]; acetone was used as a solvent instead of ether. APMI (Тm = 151-153℃) was used in experiments. According to elemental analysis, the contents of elements in APMI are as follows: C, 67.41 (calcd., 66.98); H, 4.20 (calcd., 4.19), and N, 6.35% (calcd., 6.50). The chemical shifts (δ, ppm) and multiplicity of the 13C NMR spectrum of maleimides are given in Table 1.The structures of the monomers and polymers are schematically shown on Scheme 1.
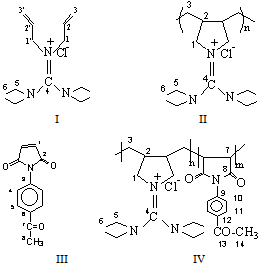 | Scheme 1. Structures of AGC (I), PAGC (II), APMI (III) and copolymer AGC-APMI (IV) |
2.2. Copolymerization
- Copolymerization of AGC with APMI was conducted in bulk and in organic solvents in the presence of 3.0 · 10-2 mol L-1 AIBN. Copolymerization was studied for a molar fraction of AMI from approximately 0.8 to 0.2in the feed. The kinetic investigations were carried out at initial conversions by the gravimetric method at 60-90℃. The reaction was allowed to go on for less than 10% conversion therefore it was terminated stopped at a certain time. Copolymers were precipitated and purified by three-fold reprecipitation by a precipitant (water) from the solution. The purified copolymers were dried under vacuum at 50℃ until constant weight was achieved. The copolymer composition was calculated from the elemental analysis data.Effective reactivity ratios r1 and r2 were calculated via the Mayo–Lewis[21], Finemann–Ross[22], and Kelen– Tüdös [23] methods.
2.3. Measurements
- The IR spectra were obtained on a IFS 66/S Bruker spectrometer in KBr tablets.The 1H and 13C NMR spectra were recorded on a "Bruker AM-300" spectrometer operating at 300 and 75.47 MHz using a broad-band proton suppression and in a JMOD mode. DMSO-d6 was used as solvent; tetramethylsilane(TMS) was used as internal standard.The calorimetric measurements were performed on DSC unit Mettler-Toledo with a scan rate of 10 or 30 ℃·min-1.The thermogravimetric and differential thermal analyses(TG/DTA) were carried out using TGA/DSC1 unit Mettler-Toledo at the heating rate of 10℃·min-1.Acute toxicity of the copolymers was measured in mongrel white male mice weighing 18-20 g, using intraperitoneal doses. The mice was injected with these copolymers. The dose was up to 1000 mg·kg-1. Each group consisted of six animals. The animals were observed for 48 h. LD50 values were calculated by Prozorovskiy’s method[24].Microbiological tests were performed by serial dilution of preparations in meat-peptone broth followed by inoculation of meat-peptone agar. Test cultures were Staphylococcus aureus strain 906, Staphylococcus saprophyticus, ATCC 15305, Micrococcus luteus, ATCC 4698, Escherichia coli strain 25922, Bacillus subtilis ATCC 6633, Salmonella euteriditis, Pseudomonas fluorescens NCIMB 9046. Bacteria were grown for 20 h or 7 days. Microbial loads were 2.5·105 cells in 1 ml of preparation-containing liquid growth medium.
3. Results and Discussion
3.1. Copolymerization of AGC with APMI
- The AGC with APMI copolymers were prepared by radical copolymerization in bulk and in DMFA at 80℃ in the presence of AIBN as the radical initiator.
|
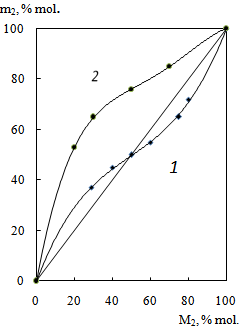
- The IR spectra of AGC with APMI copolymer (50 mol% APMI) contain several characteristic bands that are useful to the structural characterization of the polymers. We observe characteristic aliphatic methylene stretches between 3100 and 2850 cm-1 and maleimide C=O stretches are seen at 1773 and 1701 cm-1. The broadened carbonyl band at 1701 cm-1in copolymer is the result of spectral overlap between the maleimide C=O stretch and the acetyl C=O stretch.The structure of the copolymer obtained was investigated using both 1H and 13C NMR. In 1H NMR spectrum of copolymer 2H (CH-CH), 3H (CH3) of maleimide unit appeared at δ 3.85 (m) and 2.59 (d) ppm, respectively. The peaks observed at δ 3.25 and 1.14 ppm are assigned to protons of -N(-СН2-СН3)2 group. The peaks at δ 1.72, 1.58, 1.4 ppm are assigned to protons in pyrrolidinium ring and protons of CH2 group connected with APMI.Chemical shift values and signals multiplets of the atoms in 13C NMR spectrum of copolymer is presented in Table 1. The characteristic peaks observed in this study were similar to those for the copolymers obtained by copolymerization of AGC with N-substituted maleimides[17]. The peaks at 26.89 and 197.45 ppm in the spectrum are attributed to the carbons of acetyl group.As it was shown in[17], AGC and N-substituted maleimides represent monomer pairs with a strong tendency toward radical alternating copolymerization.Figure 1 shows the composition of copolymers of AGC with APMI versus the composition of initial mixtures.
|
3.2. Kinetic Regularities of Copolymerization
- The study of AGC-APMI copolymerization showed a strong dependence of reaction rate on the monomer ratio. The rate of copolymerization of AGC(М1) with APMI reduces with the increasing of molar fraction of AGC in the initial monomer mixture(Figure 2).
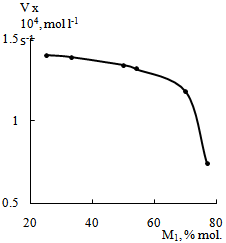 | Figure 2. Rate of copolymerization of AGC (M1) with APMI vs. monomer ratio. DMFA, [М1 + М2] = 1.4 mol·L-1, [AIBN] = 0.03 mol·L-1, Т = 80℃ |
3.3. Thermal analysis
- The thermal behaviour of the synthesized copolymers was investigated by TGA and DSC. It is known that glass transitions of polymaleimides are difficult to observed because they exhibit low changes in heat capacity [26] and are very broad. At heating rate of 10°C min-1 the copolymer showed broad scarcely noticeable glass transition. That is why the scan rate of 30°C min-1 was used. The Tg values for the AGC-APMI copolymer (66 % mol. APMI) is 222°C (Figure 3). The Tg values for the AGC-APMI copolymers range between 175 and 228℃. Tg of copolymers increases with the APMI content in the copolymer.
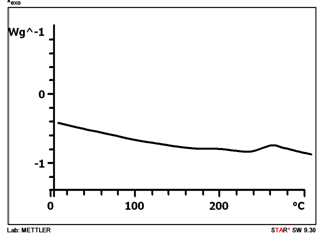 | Figure 3. DSC curve of the AGC-APMI copolymer (66 % mol. APMI) |
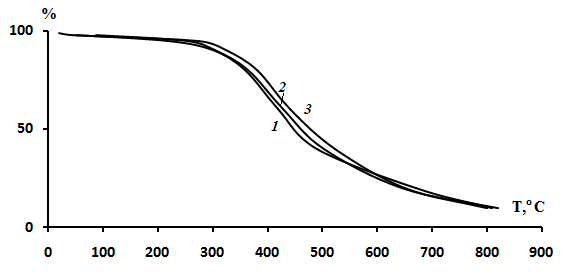 | Figure 4. TG curves of the AGC-APMI copolymers: 1 – 37 % mol. APMI; 2 – 52 % mol. APMI; 3 – 52 % mol. APMI |
| ||||||||||||||||||||||||||||||||
3.4. Biological activity
- Antimicrobial agents are defined as those materials capable of killing pathogenic micro-organisms. The great limitations of low molecular weight compounds are based on their residual toxicity even when suitable amounts of the agent are added[27]. The use of antimicrobial polymers prevents the limitation of low molecular weight analogues: reduces the residual toxicity and increases efficiency and selectivity of the agents. Moreover antimicrobial polymers are chemically stable and do not permeate through skin.Copolymers AGC with APMI were found to be nontoxic (the LD50 values were more 1000 mg·kg-1) and therefore could be used for medical purposes. The studies on antibacterial activity showed that copolymer has a pronounced antimicrobial activities with respect to Gram positive microflora. According to the results obtained the minimal bacteriostatic concentration of the copolymer with respect to Staphylococcus aureus and Micrococcus luteus is observed at 15.6 μg·ml-1, with respect to Staphylococcus saprophyticus – at 31.2 μg·ml-1 of copolymer.
4. Conclusions
- Copolymers of2,2-diallyl-1,1,3,3-tetraethylquanidiniumchloride with N-(4-acetylphenyl)maleimide have been prepared by free-radical copolymerization in dimethylformamide and in bulk at 80℃. The reactivity ratios of the copolymers were estimated using linear graphical Mayo-Lewis, Fineman-Ross and Kelen-Tüdös methods. For the system the rAPMI values are higher than the rAGC values this fact confirms the higher reactivity of APMI compared with that of AGC. The TGA results for the copolymers show that they are stable at temperatures up to 300℃. The copolymer obtained has a pronounced antimicrobial activities with respect to Gram positive microflora.
ACKNOWLEDGEMENTS
- Financial support by the Russian Foundation for Basic Reseach (grant № 11-03-96003-r_ural) and Government Research Program (kontrakt № 11.519.11.2033) is gratefully acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML