-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2012; 2(3): 35-38
doi: 10.5923/j.ajps.20120203.03
Thermodynamic and Polarization Parameters of Dibenzo-18- crown- 6 in Mixed Methanol Water Solvents
Esam A. Gomaa
Chemistry Department , Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
Correspondence to: Esam A. Gomaa , Chemistry Department , Faculty of Science, Mansoura University, Mansoura, 35516, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The different thermodynamic parameters for the solvation (Free energies, enthalpies, and entropies) ( ΔGs, ΔHs &TΔSs) for dibenzo–18–crown–6 in mixed methanol–water solvents were calculated form the experimental solubility data at different temperatures. From the experimental refractive indices and densities for the saturated solutions, the total polarization (Pt), polarizability (α), induced dipole moment (μ) and the contribution of the electronic permittivity (ε∞) were evaluated. The solvation behavior of the crown ether used in these media was discussed in view of the estimated refraction and polarization parameters.Since dibenzo 18-crown-6 has a wide applications in analytical, industrial and biological ones, then the aim of this study is to explain its solvation behaviours in methanol.
Keywords: Crown Ether, Solvation, Thermodynamic Parameters, Polarization, Mixed Methanol Water Solvents
1. Intoduction
- In recent years, macrocyclic ligands have been used in ion chromatography as components of both the stationary and mobile phases. The interest in these molecules derives from their selectivity in binding specificcations. Some labs focused on the use of macro-cycles in ion chromatography in the separation of both cations and anions .Nakagawa et al. investigated the use of crown ethers in the mobile phase for the separation of amine cations, peptides, and sulfonic acids. They showed that the increase in capacity factors is related to the stability of the complex for those compounds that form complexes with the crown ether. They also showed that the concentration of crown ether influences the capacity factors for some compounds. Peak resolution between mono- and divalent cations using an unmodified silica gel column . The improved resolution is due to adsorption of 18-crown-6 onto the stationary phase and the formation ofmacrocycle-cation complexes with selected metal ions . The logK values indicate those metal ions that bind 18-crown-6 preferentially .Recently, Dionex developed the Ion Pac CS15 analyticalcolumn, which like its precursor the CS12A,contains phosphonate and carboxylate functionalgroups, but in addition contains 18-crown-6 groups[1-5].Macrocyclic polyethers (crown ethers) are a family of compounds which possess the ability to transport ionic species across natural and artificial membranes. Because of this characteristic, they have wide applications in industry and are being investigated for their potential as pharmacologic agents.However, these compounds are highly cytotoxic in both prokaryotes and eukaryotes. Because of the cytotoxicity of crown ethers, an investigation of the potential genotoxicity of these compounds in Salmonella typhimurium was used[6].Crown ethers are very important, especially in application in thermodynamic studies[7], solvent extraction of divalent and monovalent ions[8], selective synergistic solvent extraction[9] and competitive complexation of some alkaline earths and transition metal ions[10]. Synthesis, characterization, crystal structure if some salts with dibenzo–18–crown– 6 and dibenzo–24–crown–8 macromolecules[11] are valuable ones. Therefore, studying the physical properties of the crown ethers, especially dibenzo–18–crown–6 are wanted to follow their behaviour in solutions like, apparent molar volumes and adiabatic compressibility[12].The aim of this work is to evaluate the solvation, thermodynamic and polarization parameters of dibenzo–18– crown–6 in mixed methanol–water solvents at different temperatures.
2. Experimental
- Dibenzo–18–crown–6 (DB18C6) was supplied from Merck. Methanol of BDH was used. Saturated solutions of DB18C6 were prepared by shaking 10 ml of different percentages of methanol and water in 15 ml glass tubes containing excess of the solid substance in a thermostatic water bath of the type Assistant 3193 at time intervals for a period of 2–3 weeks at 25, 30, 35 and 40℃. The molal solubility's of DB18C6 were measured (gravimetrically) by drying 1 ml of the saturated solutions in small aluminium dishes or smallbeakers under a tungsten lamp to avoid scattering of the solutions as explained by Gomaa[13,14] .The molal solubilities of DB18C6 in mixed MeOH–H2O solvents were also confirmed by spectrophotometric measurements using (Unicom UV2–100 UV/ visible spectrometer V 3.32 ) by dissolving the above residues after evaporation in pure methanol and measuring the absorption at wave length (λ) of 275 nm done using different concentrations of DB18C6 in methanol mixture at the same wave length, small differences were observed in both measurements, there for the mean values were listed in Table 1.The solubilities were read by the use from the standard curve. The refractive indices of the saturated solutions of DB18C6 were measured at 25, 30, 35 and 40℃ using a refractometer ATAGO 3T No.52507, 1 ml weighing bottle was used for measuring the densities of the saturated DB18C6 in mixed MeOH–H2O solvents at different temperatures which used for calculating the molar volume by dividing the molecular weight by density values. The heat of evaporation for solid DB18C6 was done by using DSC (Differential Scanning Calorimeter) apparatus of the type Mettler TA 3000, DSC25, made in Swizzerland.
3. Results and Discussion
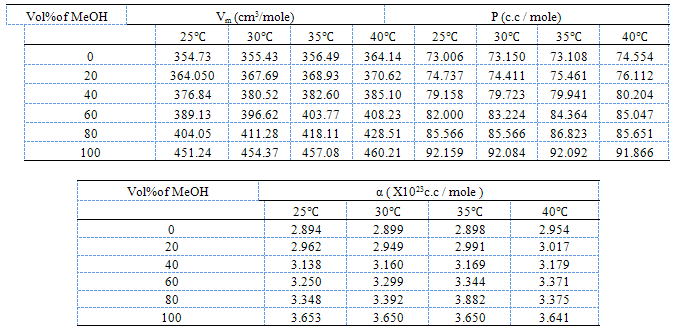
- The molal solubilities of DB18C6 were determined as reported in the experimental part at 25, 30, 35 and 40℃ in MeOH–H2O mixtures with energy of ±0.001 g/L. Their solubility are shown in Table(1) which recorded from the average of minimum experiments with experimental error of ± 0.01The solvation free energies for neutral compounds like tetraphenyl methane (Ph4C), tetra phenyl germanium (Ph4Ge)[8,9] DB18C6 in mixed MeOH–H2O solvents were calculated from the molal solubility[10] by using the following equation (1):
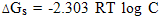 | (1) |
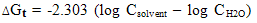 | (2) |
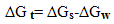 | (3) |
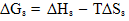 | (4) |
|
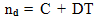 | (5) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
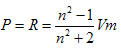 | (6) |
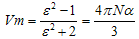 | (7) |
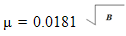 | (8) |
|
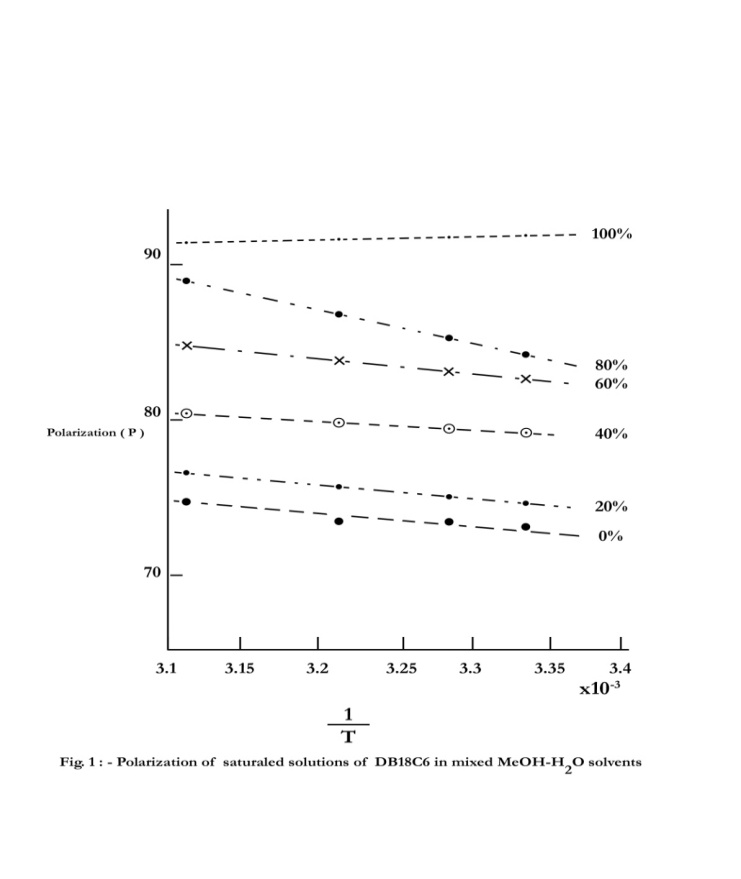 | Figure 1. Polarization of saturaled solutions of DB18C6 in mixed MeOH-H2O solvents |
|
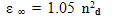 | (9) |
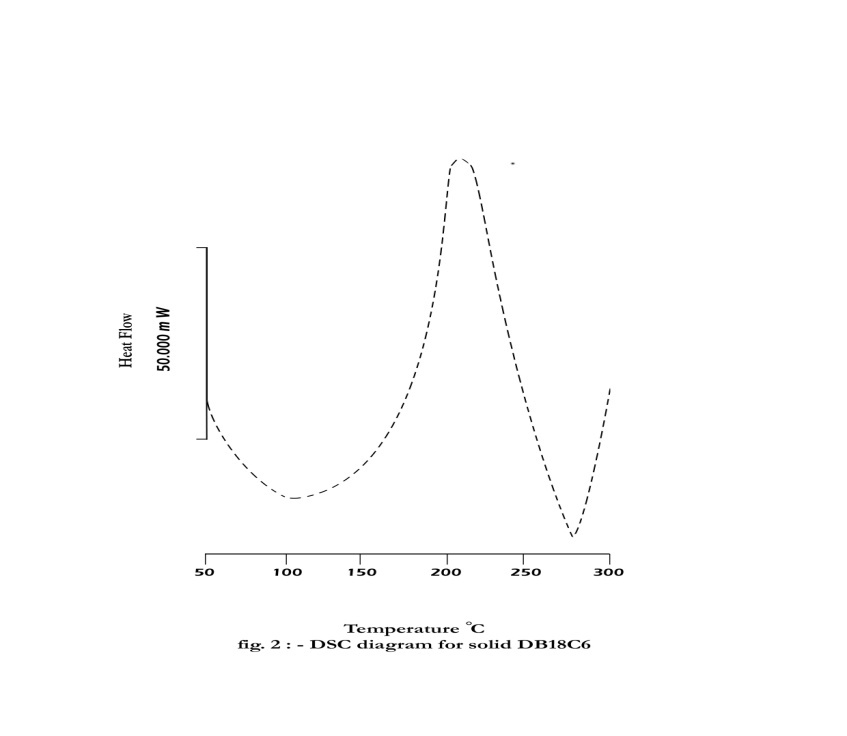 | Figure 2. DSC diagram for solid DB18C6 |
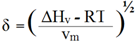 | (10) |
4. Conclusions
- All the solubility and thermodynamic parameters for dibenzo-18-crown-6 are increased by increasing methanol percentage in the mixed methanol-water solvents. This work give a lot of data about this wide range polycompound for helping in its uses and applications.
References
| [1] | Dean,A.Richens,David Simpson,Sarah Peterson, Arlo Mc Gim and John D.Lamb, Journal of Chromatography A,1016(2003) 155. |
| [2] | K.Ohta and K.Tanaka,Anal.Chim.Acta,38(1999) 265. |
| [3] | S.Kwon,K.Lee,K.Tanda and K.Ohta J.Chromatogr.,A,850 (1999)79. |
| [4] | T.Okada,J.Chromatogr.,A 758(1997)29. |
| [5] | L.R.Sousa , D. H .Hoffman, L. Kaplan and D .J .Cram , J. Am. Chem. Soc., 96(1974) 7100. |
| [6] | P.Arenaz,L.Bitticks , K.Pannell and S . Garcia, Mutagenesis.4,6,(1989) 438. |
| [7] | Takeda, Yasuyuki ; Kamazawa, Masaomi; Katsuta Shoichi, Anal. Sci, Vol. 6 (2000) 929 . |
| [8] | Dankova Marcela Makrlik, Emanueli; Vanura, petr, Models Chem., Vol. 137 (2000) 133. |
| [9] | Bond, Andrew; Dietz, Mark L; Chiarizia Renato, 1nd Eng. Chem. Res., Vol., 39 (2000) 3442 . |
| [10] | Shamsipur, M., Madrakian, T, Polyhedron, Vol. 19 (2000)1681. |
| [11] | Pasti, poola; Matijasir, Ivanko, Tusek- Bozic, lijerka, Inorg. Chim. Acta, Vol. 282 (1998) 76. |
| [12] | Bernal, Pedro; Bunn, Abet; Logam, Jennifer, Me Luann, Jennifer, J. Solution Chemistry, Vol. 29 (2000) 651. |
| [13] | J. I. Kim, A. Cecal, H. J. Born, E. A. Gomaa, Z.Phys. Chemi Neue folge, 110 (1978) 209. |
| [14] | Esam A. Gomaa, Bull – Soc. Chim. Fr, 1 (1988) 56. |
| [15] | Esam A. Gomaa. Thermochim . A cta, 147 (1989) 343. |
| [16] | J. J. Christensen, J.O. Hill and R. M. Izatt, Science, 174(1971) 459. |
| [17] | G.J. Mody and J.D.R. Thomas, "Dipole moments in inorganic Chemistry ", The University of Aberdeen Press, U. K. (1971). |
| [18] | E. A. Moelwyn Hughes "Physical Chemistry" Pergamon Press, London, (1965). |
| [19] | Raymond B. Seymour and Charles Elarraher, "Polymer Chemistry ", Marcel Dekker, New York (1992). |
| [20] | E.A.Gomaa and B.M.Al-Jahdali,American Journal of Fluid Dynamics, 1(1),2011,4-8. |
| [21] | Nagah A.El-Shishtawi,Maany a.Hammada and Esam A.Gomaa, Physical Chemistry, 1(1) ,(2011) 14. |
| [22] | E.A.Gomaa,Analele Universitate din Bucuresti-Chimie, vol. 19,(2010) 45. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML