-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2012; 2(3): 22-27
doi: 10.5923/j.ajps.20120203.01
Synthesis and Characterization of Copolymers of Methyl Methacrylate and 2-Ethoxyethyl Methacrylate
M. Manju 1, M. K. Veeraiah 2, S. Prasannakumar 3, N.M. Made Gowda 4, B.S. Sherigara 5
1Department of Chemistry, Sri Krishna Institute of Technology, Bangalore, India
2Department of Chemistry, Sri Siddhartha Institute of Technology, Tumkur, India
3Clov Chem. India Private Limited, Harohalli, Bangalore, India
4Department of Chemistry, Western Illinois University, One University Circle, Macomb IL, 61455, USA
5Department of Industrial Chemistry, Kuvempu University, Shankaraghatta, Shivamogga, India
Correspondence to: M. K. Veeraiah , Department of Chemistry, Sri Siddhartha Institute of Technology, Tumkur, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The free-radical initiated copolymerizations of methyl methacrylate (MMA) with 2-ethoxyethyl methacrylate (2-EOEMA) were carried out using 2,2-azo-bisisobutyronitrile (AIBN) as the initiator in 1,4-dioxane solutions. The copolymer products were characterized by IR, 1H-NMR, and 13C-NMR spectroscopic techniques. The reactivity ratios of the monomers were computed by the Fineman-Rose (F-R) and Kelen-Tudos (K-T) methods at lower conversion, using the IR data. The results are in good agreement with each other and the mean reactivity ratios for MMA/EOEMA copolymers are r1=0.8436, r2= 0.7751, and r1r2 = 0.6614. The reactivity ratios indicate the formation of random copolymers, which have been supported by the azeotropic composition evaluations. The distribution of monomer sequence along the copolymer chain has been calculated using a statistical method based on the reactivity ratios. The probability of finding the mean sequence lengths of 2-EOEMA and MMA units has also been evaluated.
Keywords: Methyl Methacrylate, 2-Ethoxyethyl Methacrylate, 2,2-Azo-Bis-Isobutyronitrile, Reactivity Ratio, Co-Monomer Probability Sequence, IR, NMR
Article Outline
1. Introduction
- Free radical copolymerization is a valuable tool for tailoring the properties of polymeric materials for a broad range of applications. For statistical copolymers, the monomer sequence distribution and compositional heterogeneity in and among the copolymer chains have a profound influence on the behavior and physical properties of the materials[1-7].The acrylic monomer, methyl methacrylate (MMA; CH2=C(CH3)COOCH3) has been widely used for applications in various fields[8-12]. The MMA based polymers have good biocompatibility, low toxicity, good film forming and adhesive characteristics[13]. It is also used as a comonomer in surface-active copolymerization mainly because of its good amphilic character[8-9]. Methyl methacrylate as a monomer contains a highly polar ester group, which confirms the hydrophilic nature while the methylene and methane groups in the main chain and side chain support the hydrophobic nature, respectively[10]. Methyl methacrylate and acrylate based copolymers are extensively studied by many investigators in biomedical applications such as membranes for ultra filtration/drug delivery, contact lenses and anticoagulant films[13]. Another alkene monomer, 2-Ethoxyethyl methacrylate (2-EOEMA; H2C= C(CH3) CO2CH2CH2OC2H5) is a dual functional group monomer, which has both ether and ester groups as compared to most of the vinyl acrylic monomers; these groups not only impart flexibility into the polymer, but also improve its process ability and handling[14] and improve compatibility in blends due to hydrogen bonding[15]. Because of its soft and flexible nature, 2-EOEMA based copolymers and terpolymers could be a better candidate as surfactant[16,17].The chemical structure of a co-polymer depends on the two-monomer units, which are distributed along macromolecular chains. This distribution is a direct consequence of each monomer’s reactivity in the polymer molecules[18]. Determination of the monomer reactivity ratios with small confidence intervals requires sensitive analytical techniques, careful planning of experiments, and the use of statistically valid methods of estimation[19-20]. In order to calculate the rate of polymerization or polymer productivity and copolymer composition, monomer reactivity ratios must be known[19]. Reactivity ratios were determined by F-R[21] and K-T[22] methods using the data obtained by IR analysis. The present investigation describes the synthesis of the MMA/EOEMA copolymer by free-radical polymerization and the polymer structure was characterized by IR, 1H-NMR and 13C-NMR spectroscopic techniques. Reactivity ratios calculated using the IR data are in good agreement with each other.
2. Experimental
2.1. Materials
- Methyl methacrylate (MMA), 2-ethoxyethyl methacylate (2-EOEMA), and 2, 2-Azo-bis-isobutyronitrile (AIBN) were obtained from Sigma-Aldrich Chemical Co., USA. Both MMA and 2-EOEMA, were washed with a dilute aqueous alkali solution (5% NaOH) followed by water, dried over anhydrous CaCl2 in a desiccator and stored below 00C. The organic solvents such as acetone, chloroform, tetrahydrofurane, dimethylsulfoxide, dimethylformamide, dimethylacetamide, ethyl acetate, isobutyl acetate, benzene, hexane, and 1, 4-dioxane were of AR Grade with 99 % purity. They were used as received from E. Merck Chemicals, Mumbai, India.
2.2. Synthesis
- Copolymerization reactions of varying compositions of monomers[MMA/EOEMA: (A) 25:75, (B) 33.3:66.7, (C) 50:50, (D) 66.7:33.3, (E) 75:25] were carried out in 1,4-dioxane solvent at 60℃ under the inert nitrogen atmosphere (Scheme I) For each reaction, calculated amounts of 2-EOEMA, MMA, 1,4-dioxane and 0.5% AIBN were taken in a 250 mL three-necked round bottom flask, placed in an oil bath maintained at 60℃ with continuous stirring. The copolymerization was allowed to proceed to high conversion (less than 15%) under nitrogen atmosphere. The polymerization reaction was stopped by cooling the reaction mixture to room temperature. The polymer product obtained was precipitated in a diethyl ether/hexane mixture to ensure complete removal of residual monomers. Copolymer samples were suction-filtered and dried in vacuum at 40℃ until constant weight.
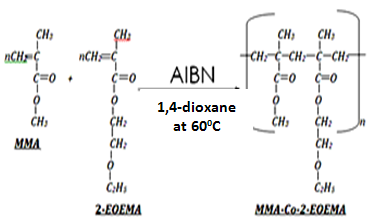 | Scheme 1. General reaction for the synthesis of MMA/EOEMA copolymer |
2.3. Solubility Measurement
- Solubilities of polymers were tested in polar and non-polar organic solvents. To a 5.0 mL solvent, a known amount of the copolymer sample (5-10 mg) was added and kept stirred for 24 hr. The observations showed that the copolymers were completely soluble in most of the polar solvents such as acetone, chloroform, tetrahydrofurane, dimethylsulfoxide, dimethylformamide, dimethylacetamide, ethyl acetate, isobutyl acetate, and 1,4-dioxane. They were insoluble in non-polar solvents like hexane, cyclohexane, carbon tetrachloride, and benzene and in highly polar water.
2.4. Spectroscopic Analysis of Copolymers
- Copolymers were characterized by: i) Fourier-Transform infrared (FT-IR) spectra in acetone recorded on a Simadzu – 1800S spectrometer at 2 cm-1 resolution with 64 scans over the spectral range of 4000-400 cm-1; ii) Proton-NMR) and 13C-NMR spectra in CDCl3 or DMSO-d6 as solvent on a Bruker AMX-400 spectrometer using tetramethylsilane (TMS) as the internal standard at the Indian Institute of Science, Bangalore, India.
3 Results and Discussion
3.1. Copolymers characterization
- Copolymers of MMA/EOEMA having different compositions were prepared using AIBN as a free-radical initiator in 1,4-dioxane solutions under nitrogen atmosphere under 60℃.
3.1.1. Infrared Spectral Study
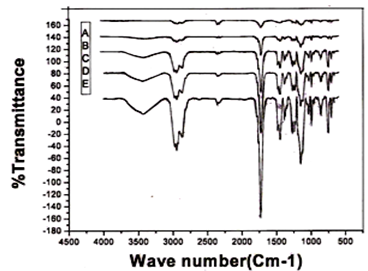
- Infrared spectra of the new copolymers are presented in Fig. 1. A strong absorption peak at 1731.7 cm-1 is due to the carbonyl ester group of MMA and 2-EOEMA units of the polymer. The peak in the range, 1125.4 cm-1 - 1271.3 cm-1, is due to a strong absorption caused by an asymmetric C-O-C stretching mode in the vinyl ether of 2-EOEMA unit. The peak at 995.95 cm-1 is due to the alkene (C2H4) group of the 2-EOEMA unit. The peaks at 1386.18 cm-1 and 1451.56 cm-1 are due to the Alkyl (-C-H) group of both MMA and 2-EOEMA units. Peaks at 1766.75 cm-1 and 1789.59 cm-1 are due to ketone (C=O) group of both MMA and 2-EOEMA units.
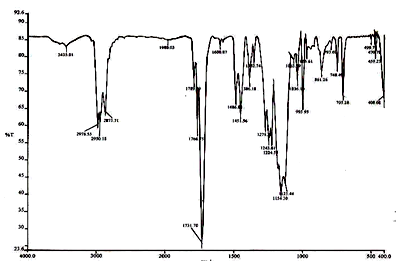 | Figure 1. IR Spectrum of MMA/EOEMA copolymer |
3.1.2. Nuclear Magnetic Resonance Spectroscopy
- The 1H-NMR spectrum of a copolymer product is shown in Fig. 2. The main methylene proton signals of both the MMA and 2-EOEMA units resonate at ∂ 2.09–1.89 ppm, which overlap with the type of compositional and configurational sequences. Hence, it is difficult to assign these. Similarly, methyl proton signals of 2- EOEMA observed at ∂ 0.88–1.29 ppm are overlapping with each other. Two (–CH2 – C = O) proton signals of the keto group of both MMA and 2-EOEMA are observed at ∂ 2.79 ppm while a multiplet of three methylene (CH2, CH2,CH2) protons for 2-EOEMA appear at ∂ 3.5 – 3.69 ppm. The absorption of methylene protons (–CH2–O–CH2–) of the ether group of 2-EOEMA occurs at ∂ 4.12 ppm. The multiplet for protons of conjugated olefins of the copolymer resonates at ∂ 7.35–8.10 ppm, whereas the methyl ester group protons of MMA (COO-CH3) units resonate around 3.35 ppm, respectively. The structure of the copolymer was further confirmed by 13C-NMR spectroscopy (Fig. 3). The carbonyl carbon (>C=O) signals of both the MMA and 2-EOEMA units are appearing at ∂ 205.2 ppm. The signals of ester group carbons (R-COO-R1) of both the MMA and 2-EOEMA units appear at ∂ 162.8 ppm. The olefinic carbon absorptions of 2-EOEMA appearing in the region, ∂ 134.7 ppm – 125.5 ppm are quite complex. The ether (C - O - C) group carbons of 2-EOEMA occur in the region, ∂ 67.4 ppm – 64.1 ppm, whereas carbon absorptions of the – CH and -O-CH3 groups of MMA and 2-EOEMA appear at ∂ 51.14 ppm. The side chain methylene carbons of both the MMA and 2-EOEMA absorb at ∂ 29.48 ppm – 28.34 ppm. The spectral region of ∂ 67.63 ppm – 14.76 ppm is quite complex and overlapping may be due to aliphatic carbon resonance in the backbone and side chain of the copolymer. Furthermore, two methyl carbons of 2-EOEMA resonate at ∂ 14.76 ppm.
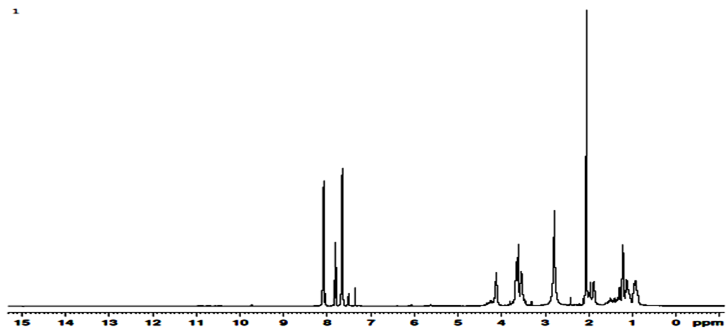 | Figure 2. 1H-NMR Spectrum of MMA/EOEMA copolymer |
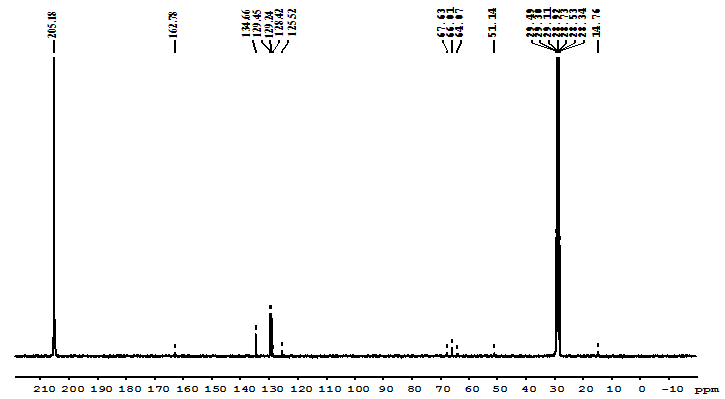 | Figure 3. 13C-NMR Spectrum of MMA/EOEMA copolymer |
3.1.3. Copolymer Composition
- The composition of monomers in the polymer product was determined by IR spectroscopy through recorded absorption bands for comonomers[23,24]. Pure carbonyl group of the MMA/2-EOEMA copolymer has a characteristic, broad infrared absorption band at 1732 cm-1, while the ether group of 2-EOEMA is at 1152 cm-1. These carbonyl absorbance peaks in the copolymers of different composition are shown in Fig. 4. It is evident that with an increase in 2-EOEMA content, peak-1 increases correspondingly. Using the absorbance of carboxyl groups of MMA and EOEMA of all copolymer compositions, the concentrations of 2-EOEMA and MMA in the copolymer were obtained. The product compositions obtained by the IR method were analyzed and the results calculated according to the following equations[25].
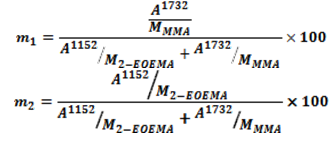
 | Figure 4. FT-IR spectra of MMA/2-EOEMA copolymers of different compositions of MMA/EOEMA: (A) 25:75, (B) 33.3:66.7, (C) 50:50, D) 66.7:33.3, (E) 75:25 |
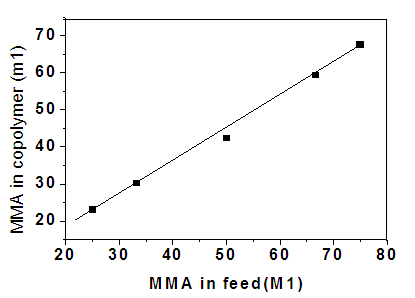 | Figure 5. Plot of mol% MMA (M1) in the reaction mixture vs. mol% MMA (m1) in copolymer |
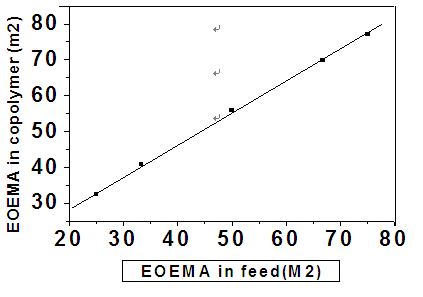 | Figure 6. Plot of mol% of 2-EOEMA (M2) in the reaction mixture vs. mol% 2-EOEMA (m2) in copolymer |
3.1.4. Monomer Reactivity Ratio
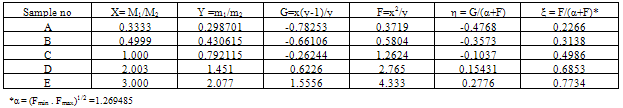
- The comonomer composition sequence is one of the main factors that influences copolymer behavior and properties. The copolymer composition depends on the monomer feed composition and on the relative monomer reactivity. Therefore, it is very important to study the comonomer reactivity in these systems[26]. Copolymerization reactivity ratios of MMA and 2-EOEMA in the products were determined using the standard Fineman-Rose (F-R)[21] and Kelen-Tudos (K-T)[22] methods based on IR spectroscopy. The equations (1) and (2) were used for F-R and K-T methods, respectively.
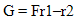 | (1) |
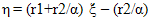 | (2) |
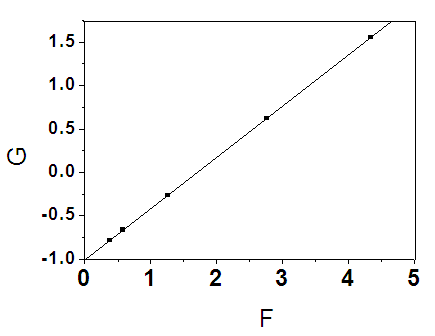 | Figure 7. F-R plot of G vs. F |
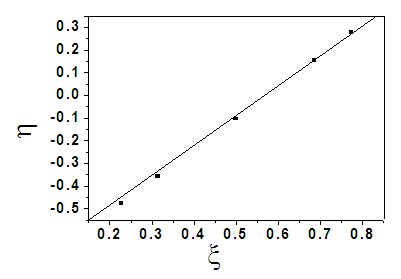 | Figure 8. K-T plot of . η vs. ξ |
3.1.5. Azeotropic Composition
- The shapes of the azeotropic compositions of the copolymer systems and distribution of monomer units are random. The azeotropic composition (N1) was determined by equation (3).
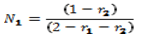 | (3) |
3.1.6. Copolymer Microstructure
- The copolymer microstructure and sequence distribution of monomers in the formation of the copolymer were evaluated. The probability of finding the sequence of n EOEMA and n MMA units is calculated using the following equations:
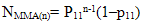 | (4) |
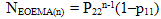 | (5) |
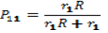 | (6) |
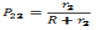 | (7) |
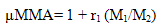 | (8) |
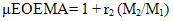 | (9) |
|
|
|
4. Conclusions
- Copolymers of MMA and 2-EOEMA were synthesized using AIBN as a radical initiator in solution. The copolymers obtained show good solubility in most of the polar solvents and insoluble in non-polar solvents. The characterization of copolymers performed by IR, 1H-NMR and 13C-NMR techniques confirms the chemical structure and composition.The reactivity ratios determined by two standard methods using IR data are in good agreement with each other. Calculated reactivity ratios indicate that 2-EOEMA is more reactive than MMA. The monomer sequence distributions calculated from the statistical method also indicates the copolymer formation with predominately-random distribution of the monomers with a higher content of 2-EOEMA units in the polymer chain. However, reactivity of the monomer depends on its strength and nature of the functional groups present.
References
| [1] | Moad, G.; Solomon, D.H. The Chemistry of Radical Polymerization, 2nd ed.; Elsevier: Amsterdam, 2006 |
| [2] | El-Newehy, M.H.; Al-Deyab, S.S.; Al-Hazmi, A.M.; 2010, Reactivity Ratios for Organotin Copolymer Systems. Molecules, 15, 2749-2758 |
| [3] | Debashish, R.; Aman, U.; Brent S. Suin. J.; 2009, Macromolecules, 42 (20), 7701–7708 |
| [4] | Phillip, D.H.; Roger, L.K.; Daniel, J.A.; Edmund, M.C.; Timothy, T.W., 2007, Macromolecules,40 (20), 7061-7064 |
| [5] | Xia, T.; Guang, W.; Armand, S.; Yue, Z., 2005, J. Phys.Chem. B, 109, 20281-20287 |
| [6] | Gallado, A.; Lemus, A.R.; San, R.J.; Cifuentes, A.; Diezmasa, J.C., 1999, J. Macromolecules, 32,610-617 |
| [7] | Brar, A.S.; Kumar, R. J.; 2002, Polym.Sci., part A: poly.Chem., 40, 2225-2236 |
| [8] | Radic, D.; Gargallo, L.; 1997, J. Macromolecules, 30,817 |
| [9] | Khosrou, A.; Ali M.; Mohsen A.; Mozhgan, B.; Farideh, G.P.; 2005, Iranian Journal of Pharmaceutical Research, 4: 227-232 |
| [10] | Bhattacharya, A.; Misra, B.N.; 2004, Prog. Polym. Sci., 29, 767–814 |
| [11] | De, Q.A.; Vargas, R.R.; Higa, O.Z.; Ribeiro, R.R.; Vitolo, M. J.; 2002, Appl. Poly.Sci., 84, 767 |
| [12] | Eurisk Assessment-Methyl Methacrylate, CAS No: 80-62-6, EINECS No: 201-297-1, FINAL REPORT, 2002 |
| [13] | Sheng, Q.N.; Fen, R.; Chao, H.; Peng, F. Z.; Xiao, H.W.; Jie, L.; Chang, S.Z.; 2011, Chinese Chem.Letters, 22(3), 370-373 |
| [14] | Vijay, K.S.; Namdev, B.S.; Prasannakumar, S.; Sherigara, B.S.; Aminabhavi, T.M.; 2011, Journal of Polymer Research, 18(3), 359-366 |
| [15] | Seniha,G.F.; Yusuf,Y.; A. Tuncer Erciyes.; 2006, Prog. Polym. Sci., 31, 633–670 |
| [16] | Sanmathi,C.S.; Prasannakumer,S.; Sherigara, B.S.; 2004, Bull. Mater. Sci., 27,(3), 243-249 |
| [17] | Tiemblo, P.; Laguna, M.F.; Garcı,F.; Garcı, J.M.; Riande, E Guzma´n, J.; 2004, Macromolecules, 37, 4156-4163 |
| [18] | Gatica, N.; Natali, F.; Alejandra,O.; Deodato, R.; 2005, J.Chil. Chem.Soc., 50, 581 |
| [19] | Wessling, R.A.; 1968, J. Appl. Polym. Sci., 12, 309–319 |
| [20] | Vijay, K.S.; Prasanna Kumar, S.; Musturappa, T.E.; Mahadevan, K.M.; Sherigara, B.S.; 2007,J. Macromolec. Sci., part A: Pure and Appl. Chemistry, 44, 1161-1169 |
| [21] | Gan, L.H.; Roshan, D.G.; Gan, Y.Y.; 1998, European Polymer Journal, 34( 1), 33-36 |
| [22] | Vijay, K.S.; Prasanna Kumar, S.; Sherigara, B.S.; Reddy, B.S.R.; Aminabhavi, T.M 3.; 2008, J. Macromol. Sci, part A: Pure and Appl. Chem, 45, 821-827 |
| [23] | Dong, S.; Wel, Y.; Zhang, Z.; 1999, J. Appl. Polym.Sci., 74, 516 |
| [24] | Pekel.N.; 2001, Eur. Polym. J., 37, 2443 |
| [25] | Rzaev, Z.M.O.; Guner, A.; Kibare, G.; Kalplan, H.C.; Asisi, A.; 2002, Eur. Polym. J., 38,1245 |
| [26] | Gatic, N.; Gargallo, L.; Radic, D.; 2002, Eur. Poly. J., 38, 1371 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML