-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2012; 2(2): 1-4
doi: 10.5923/j.ajps.20120202.01
One Pot Cat ionization of Cassia Gum to Prepare Gum Derivative for the Use of Personal Care Products
Omprakash H. Nautiyal
India malt Pvt. Ltd., part of noveon, Taluk Savli, Post Manjusar, Dist. Vadodara 371 995, Gujarat, India.
Correspondence to: Omprakash H. Nautiyal , India malt Pvt. Ltd., part of noveon, Taluk Savli, Post Manjusar, Dist. Vadodara 371 995, Gujarat, India..
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Cassia gum finds an extensive use in the application of foods as stabilizer, thickener, and clouding agent. But its cat ionization with various nitrogen contents has impeccable influence on its physical properties especially viscosity that exploits its usage in the personal care products. It may be used for the scalp shampoos for conditioning hair makes them lustrous, shiny, and healthy over the period of application. One pot synthesis was investigated to make the preparation commercially feasible and less process time consuming. Hence all the steps involving of preparation, washings were carried out in one pot.
Keywords: Cat ionization, QUAB, PCP, nitrogen contents, viscosity
Article Outline
1. Introduction
- The Cat ionization Process When the galactomannans are reacted with the quab then the quab is converted to dihydroxy compound and it subsequently reacts with mannose attached to the main chain of the galactomannans. As a result epoxy forms and the nitrogen attached to it retains the positive charge and due to this the process is termed as cat ionization (an electrons deficient centre). This provides the excellent viscosity in various applications. Ferdinand et al. Describes in their invention that relates to substantially pure hydrocolloids obtained from the endosperm of seeds (hereinafter “hydrocolloids”), a method of obtaining said hydrocolloids, and compositions comprising said hydrocolloids. More specifically, the present invention relates to a method for obtaining galactomannan hydrocolloids wherein the hydrocolloids are colorless, odorless, tasteless, and substantially free of anthraquinones and exhibit improved performance parameters such as increased viscosity, gel strength and break strength properties. The invention further relates to hydrocolloids obtained by the process of the invention that have been derivatized by anionic, cationic, nonionic and/or amphoteric substituents. The hydrocolloids and derivatized hydrocolloids of the invention can be employed as gelling and binding agents’ thickeners, stabilizers, emulsifiers, spreading and deposition aids and carriers for enhancing the rheology, efficacy, deposition, psycho sensory, aesthetic and delivery of chemically and physiologically active ingredients in food and fodder, personal care, health care, pharmaceutical, household, institutional and industrial compositions in which they are included.The initial step in the cat ionization process is the chemical conversion of the cat ionizing reagent from its stable chlorohydrins form to its corresponding reactive epoxide form. This conversion is achieved by reacting equimolar quantities of alkali with the chlorohydrins. The resulting epoxide can then further react, again under alkaline conditions, with the hydroxyl or amino groups on the polymeric substrate. The resulting product is a substrate with a chemically bound quaternary ammonium group, which imparts a cationic charge.Tsai et al. invention relates to polycationic reagents, to novel cationic polysaccharide derivatives produced by reaction of polysaccharides with these reagents, and to the use of these polysaccharide derivatives in paper manufacturing. The polycationic reagents have at least two cationic groups and one polysaccharide-reactive group. Certain of these reagents are novel compositions of matter. The modification of starch and other polysaccharides by chemical derivatization to produce various cationic polysaccharides is well known. Cationic polysaccharides, i.e., polysaccharides which have been modified so that they have a positive electrostatic charge, are used for a large number of applications and are particularly useful in the manufacture of paper due to their superior performance in the paper production as compared to unmodified polysaccharides. Amphoteric polysaccharides, i.e., polysaccharides which have been modified so they have cationic groups, together with a controlled amount of anionic (e.g., phosphate) groups, are used in a similar manner, with superior performance as compared to unmodified polysaccharides. Primarily the ground purified endosperm of the seeds of Cassia tora and Cassia obtusifolia, (Fam. Leguminsae) containing less than 0.05% of Cassia occidentals. It consists mainly of high molecular weight (approximately 200,000- 300,000) polysaccharides composed of galactomannans; the mannose: galactose ratio is about 5:1. The structural formula for cassia gum galactomannan (Figure 1).is given below. The seeds are de husked and de germed by thermal mechanical treatment followed by milling and screening of the endosperm. The ground endosperm is further purified by extraction with isopropanol.[1-2]
1.1. Applications
- Cationic polymers are often used as conditioners in skin and/or hair compositions. Quatemized polymers are used in shampoos and conditioners to facilitate comb ability. The positively charged nitrogen bonds with negatively charged hair fibbers to form films. They also make the hair feel softer and smoother to the touch without creating too much build-up. The poly galactomannan hydrocolloids of the invention can be used as part of a cationic polymer conditioner package in a conditioning detergent formulation that not only imparts cleansing, wet detangling, dry detangling and manageability properties to the hair, but also is relatively non-irritating. This composition is thus suitable for use by young children and adults having sensitive skin and eyes. In one embodiment of the invention, cationic cassia and cationic guar derivatives are very efficient in these applications. [1-2]A product is considered to be a conditioner if it improves the quality of the surface to which it is applied, particularly if this improvement involves the correction or prevention of certain aspects associated with surface damage. Conditioning of the hair and skin must be a continuous process, as both substrates are in a constant cycle of shedding and renewal. The main difference between hair and skin is that skin is basically a living organ that replaces its outermost layer on a frequent basis. Hair, in contrast, is basically dead material derived from a few live cells deep within the skin surface. Thus, conditioning agents for skin can affect the homeostatic processes of growth and repair by supplementing the body’s renewal mechanisms. Conditioning agents for hair have no effect on growth and cannot affect cellular repair. Rather, they can only temporarily improve the cosmetic appearance of damaged hair and must be reapplied as removal occurs. For skin to appear and feel normal, the moisture content of the upper layer must be above 10%. Moisture is lost through evaporation under low humidity conditions and must be replenished with water from the lower epidermal and dermal layers. Once skin damage has occurred and the barrier has been damaged, reconditioning can occur only if the loss of moisture is retarded. This is the goal of moisturizers, which function temporarily until skin integrity can be re- established. Hair damage results from both mechanical and chemical treatments that alter any of the physical structures of the hair. Conditioning agents cannot enhance repair, but can temporarily increase the cosmetic value and function of the hair shaft until removal of the conditioner occurs with cleansing. Most hair damage occurs as a result of grooming habits and exposure to chemicals used for esthetic purposes, such as shampooing, drying, combing, brushing, styling, dyeing, permanent waving, and to environmental factors, such as sunlight, air pollution, wind, seawater, and chlorinated swimming pool water. There are several mechanisms by which conditioners can improve the cosmetic value of the weathered hair shaft by increasing shine, decreasing static electricity, improving hair strength and protecting against ultraviolet radiation. Conditioning the hair can mitigate this hair damage by improving sheen, decreasing brittleness, decreasing porosity, and increasing strength.[1-2] Polymeric conditioners help hair and skin look and feel better by improving the physical condition of these surfaces. Hair conditioners are intended primarily to make wet hair easier to detangle and comb and to make dry hair smoother, shinier, and more manageable. Skin conditioners primarily moisturize, while providing protection from the drying effects of the sun, wind, and contact with harsh detergents. Cationic polymers are very efficacious conditioning agents because of their substantively to the respective substrate, which is directly attributable to electrostatic interactions between oppositely charged sites on the hair shaft or skin surface and on the polymer backbone. Conditioners typically remain on the fiber surface, reducing combing forces and flyaway, and in some systems, providing enhancement of volume, curl retention, body and manageability. [.3-4]
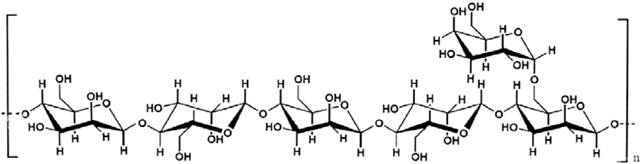 | Figure 1. Cassia gum functional structure |
2. Materials and Methods
- QUAB 151 was imported from Degussa Germany, with 70% of solution in water and was stored underground tank until in use. Cassia gum was in house product. Sodium hydroxide and hydrochloric acid was purchased from the Indian companies.
2.1. Measurement of Nitrogen
- Nitrogen content before and after cat ionization was determined by Kedah’s method as per standard procedure. [5-6]
2.2. Measurement of Viscosity of Cat Ionized Gum
- The viscosity of 1 weight % cat ionized cassia gum solution was measured by employing (Brookfield viscometer after 5 min. at 25℃. and 100 rpm).
2.3. Cat ionization Process
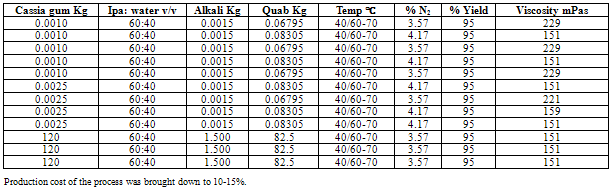
- In a 2 liter capacity of cylindrical glass reactor fixed with proper clamping and agitator there was charged 10 g of cassia gum in volume of 1 liter of IPA: water; 60:40. It was agitated properly and 1.5 g of an alkali and 67.95 g of quab was added to the reaction mass. Initially the temperature was maintained at 40℃ for 1 h and during the end of the reaction the temperature was raised to 60-70℃. After 4 h of reaction time it was cooled and washed with IPA: water mixture to get rid of depleted amines. The washes were repeated for two times more to get the amines free product. It was then filtered and was allowed to dry in the vacuum oven at 80-100℃ to dry it completely. Once it was dried then the viscosity, N2 content and the yield was calculated. (Table 1)
|
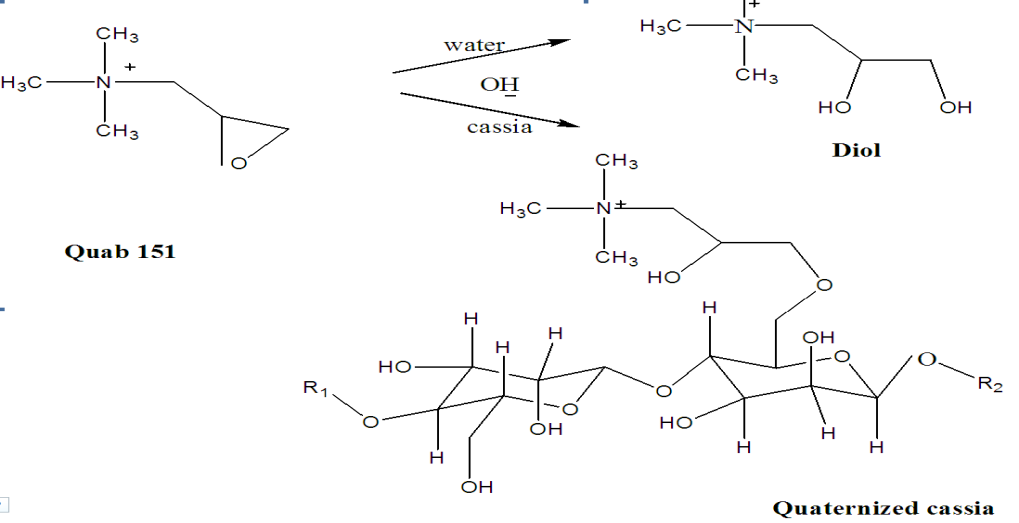 | Figure 2. Schematic Flow of synthesis |
3. Results and Discussion
- The increase of mole ratio of quab to gum yielded high nitrogen content cat ionized gum. The gum with different nitrogen content has shown the different physical properties. Cat ionized gum with 0.50mol of quab yielded the gum with 4.17% of nitrogen content; viscosity 151 mPas and with 0.43% of quab yielded the gum with 3.57% of nitrogen content and viscosity of 229 mPas. The turbidity of the solution was found to be passing. Turbidity may be poor due to un dissolved cat cassia fibers in prepared solution.Application test performed using the cat ionized gum for shampoo preparation was found to pass in its cohesive test. This research was completely reinvestigated to make the process conditions and yield feasible for the company business. The color and odor of the cat ionized gum was as per the international standards. Cat cassia gum on testing at P&G laboratory declared passing all the specifications. Homogenous phase for the cat ionization plays an important role and hence was best optimized for uniform dispersion of the reacting gum. Cat ionization has great impact on the rheological and physical properties of cat cassia as compare to cassia gum with regards to its viscosity and nitrogen contents. Viscosity of the cat cassia is proportional to the nitrogen content during the cat ionization process. Viscosity plays an important role in the final formulation of the cationic nature of shampoo. Protein contents of the cat cassia are also very cru cial from the customers’ demands point of view. (Figure 3)
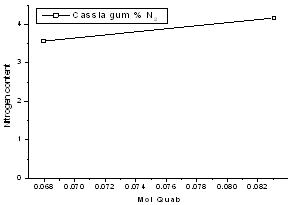 | Figure 3. Effect of QUAB mol on cassia gum |
4. Conclusions
- On changing the ratio of the isopropyl alcohol and water plays an important role in the final product’s quality, color and odor. Protein discard during the final processing also helps in acceptance of the product in the marketability. Cohesive tests performed in the application laboratory employing the cat cassia was found to be very promising than the earlier invention. Microbiological status of the cat cassia was very challenging during its preparation.Cationic polymers are often used as conditioners in skin and/or hair compositions. Quaternized polymers are used in shampoos and conditioners to facilitate compatibility. The positively charged nitrogen bonds with negatively charged hair fibers to form films. They also make the hair feel softer and smoother to the touch without creating too much build-up. The polygalactomannan hydrocolloids of the invention can be used as part of a cationic polymer conditioner package in a conditioning detergent formulation that not only imparts cleansing, wet detangling, dry detangling and manageability properties to the hair, but also is relatively non-irritating. This composition is thus suitable for use by young children and adults having sensitive skin and eyes. In one embodiment of the invention, cationic cassia and cationic guar derivatives are very efficient in these applications.
ACKNOWLEDGEMENTS
- This research was possible with the help of R&D chemists and application scientists in India and USA Brecksville. I heartily thank all of them. Dr. Arup Roy, research associate deserves special thanks for his morale support and comments.
Notes
- 1. This process was invented and developed by the author. This process was shown to demonstrate the reduction in the production cost by 10-15%. It was well appreciated by the company management. Finally the production batches were carried out in the plant. The success of the process help in expansion of the plant and now produces 250 MT/month.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML