-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2025; 13(1): 1-4
doi:10.5923/j.ajoc.20251301.01
Received: Apr. 13, 2025; Accepted: Apr. 29, 2025; Published: May 8, 2025

1-Butyl-3-Methylimidazolium Tetrafluoroborate ([BMIM][BF₄]) and Hexafluorophosphate ([BMIM][PF₆]) Mediated Synthesis of Bicyclic Lactones
Bello Makama , Abby Adams
The Department of Chemistry and Physical Sciences, Nicholls State University, 906 East First Street, Thibodaux, Louisiana, United States of America
Correspondence to: Bello Makama , The Department of Chemistry and Physical Sciences, Nicholls State University, 906 East First Street, Thibodaux, Louisiana, United States of America.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The transition toward sustainable solvent systems is essential for alleviating the environmental and safety risks associated with traditional organic synthesis methodologies. This study investigates the sodium borohydride (NaBH₄) reduction of (1S,2R,3S)-3-methyl-2-(nitromethyl)-5-oxocyclopentylacetic acid using two ionic liquids—1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF₄]) and hexafluorophosphate ([BMIM][PF₆])—as environmentally benign alternatives to ethanol. The reduction conducted at room temperature in [BMIM][PF₆] yielded a syn:anti product ratio of 74:26, whereas [BMIM][BF₄] resulted in a ratio of 69:29, in stark contrast to ethanol's significantly lower syn selectivity of 21:69, accompanied by the formation of an undesired byproduct identified as an amine from the reduction of the nitro group. At a temperature of 0°C, [BMIM][PF₆] further improved selectivity to 83:17, demonstrating enhanced control over stereochemistry. These outcomes are attributed to the distinctive solvation environment offered by ionic liquids, which inhibit NaBH₄ decomposition, enhance substrate solubility, and stabilize reactive intermediates. Furthermore, these solvents facilitate the simplification of product isolation, reduce waste generation, and eliminate risks associated with solvent evaporation. The findings highlight the potential of ionic liquids to replace volatile organic solvents, thereby fostering safer, more efficient, and environmentally sustainable practices in organic synthesis.
Keywords: Green Chemistry, Ionic Liquids, NaBH₄ Reduction, Sustainable Synthesis, [BMIM][BF₄], [BMIM][PF₆]
Cite this paper: Bello Makama , Abby Adams , 1-Butyl-3-Methylimidazolium Tetrafluoroborate ([BMIM][BF₄]) and Hexafluorophosphate ([BMIM][PF₆]) Mediated Synthesis of Bicyclic Lactones, American Journal of Organic Chemistry, Vol. 13 No. 1, 2025, pp. 1-4. doi: 10.5923/j.ajoc.20251301.01.
Article Outline
1. Introduction
- The utilization of sodium borohydride (NaBH₄) as a chemoselective reducing agent is well-documented, particularly in the conversion of carbonyl functionalities to their corresponding alcohols [1]. These reductions are typically conducted in protic solvents such as ethanol or methanol, which facilitate the solubilization of both the substrate and the reducing agent [2]. However, these solvents present environmental and safety challenges, including flammability, volatility, and the generation of hazardous waste streams [3].In response to these issues, ionic liquids (ILs) have emerged as environmentally benign alternatives. Comprising bulky organic cations and weakly coordinating anions, ILs exhibit properties such as negligible vapor pressure, high thermal stability, and non-flammability [4,5]. Their tunable solvation environments and structural diversity make them suitable mediums for a variety of chemical transformations, rendering them attractive candidates for incorporation within green synthetic methodologies [6,7]. Numerous studies have indicated that ILs not only create a safer reaction environment but also enhance selectivity, catalytic efficiency, and sustainability across a spectrum of reactions, including reductions utilizing hydride donors [8,9]. In these reactions, ILs improve substrate solubility, stabilize reactive intermediates, and mitigate decomposition pathways that are typically observed with NaBH₄ in protic solvents [10,11]. Moreover, certain ionic liquids have been shown to influence stereoselectivity, a phenomenon attributed to their polarizable and highly organized solvation shells, which can preferentially favor the formation of one diastereomer over another [12].This study investigates the reduction of (1S,2R,3S)-3-methyl-2-(nitromethyl)-5-oxocyclopentylacetic acid through the application of NaBH₄ in two hydrophobic ionic liquids, [BMIM][BF₄] and [BMIM][PF₆], compared to ethanol. The objective is to quantify variations in stereoselectivity, yield, and efficiency while also underscoring the advantages of ionic liquids as sustainable solvents for fine chemical and pharmaceutical synthesis. The broader implication of this work is the demonstration of ionic liquids as feasible substitutes for volatile organic solvents in industrial-scale reductions, thus contributing to greener and safer chemical manufacturing practices.
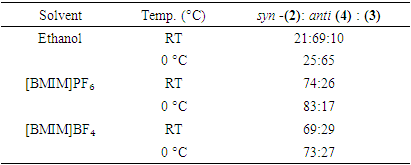
2. Results and Discussion
- The reduction of (1S,2R,3S)-3-methyl-2-(nitromethyl)-5-oxocyclopentylacetic acid (1) at 0°C utilizing NaBH₄ in ethanol, [BMIM][BF₄], and [BMIM][PF₆] yields a diastereomeric mixture of syn and anti-alcohols. However, the selectivity for the syn isomer, which readily undergoes cyclization, exhibited considerable variability. [BMIM][PF₆] produced a syn:anti ratio of 83:17, whereas [BMIM][BF₄] resulted in a 73:27 ratio. In contrast, the reduction in ethanol favored the anti-isomer, yielding a syn:anti ratio of 25:65. These results underscore the significance of ionic solvents in facilitating the formation of the desired bicyclic compound.When the reaction was repeated at room temperature for 1 hour in ethanol, TLC analysis showed the appearance of a new product. The solvent was removed under reduced pressure and the residue was subjected to gradient column chromatography, affording compounds (2), (4), and (3) in a ratio of 21:69:10, respectively. Their masses, IR, and ¹H NMR spectra fully supported the proposed structures (Scheme 1). These findings emphasize the advantageous role of ionic liquids in enhancing reaction efficiency compared to traditional protic solvents such as ethanol. It is posited that the improved yields observed in [BMIM][BF₄] and [BMIM][PF₆] are attributable to several intrinsic properties of ionic liquids. Ethanol, as a protic solvent, can partially decompose NaBH₄ through protonation, leading to decreased availability of the reducing agent [13,14]. In contrast, ionic liquids offer a non-protic, highly stable environment that prevents premature decomposition of NaBH₄ and promotes more efficient hydride transfer [15,16].
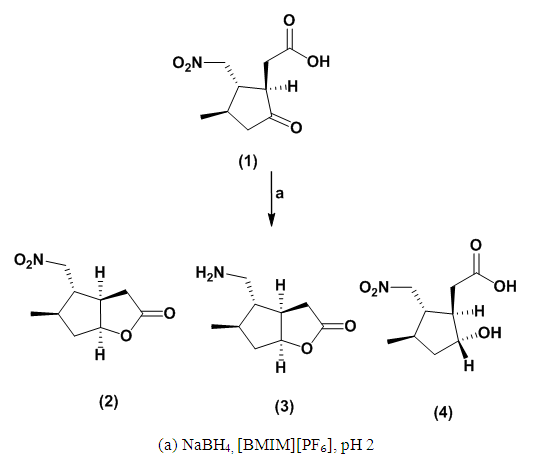 | Scheme 1. Stereoselectivity in NaBH₄-Mediated Ketone Reduction |
|
3. Conclusions
- The findings of this study strongly support the use of ionic liquids as additional good solvent option for NaBH₄ reductions in advanced organic chemistry experiments. Replacing ethanol with [BMIM]BF₄ and [BMIM]PF₆ resulted in higher reaction yields, improved stereoselectivity, and enhanced reaction control, demonstrating the transformative potential of ionic liquids in organic synthesis. Notably, the reaction in [BMIM]PF₆ exhibited the highest efficiency, yielding an 83:17 syn:anti ratio, while [BMIM]BF₄ produced a 73:27 syn:anti ratio, both significantly outperforming ethanol. The advantages of ionic liquids over ethanol in NaBH₄ reductions can be attributed to several key factors: minimized NaBH₄ decomposition—unlike ethanol, which can lead to premature hydrolysis of NaBH₄, ionic liquids provide a more stable reaction environment, ensuring greater hydride availability throughout the reaction; enhanced solubility and reaction kinetics—the unique solvation properties of ionic liquids facilitate better dissolution of both reactants and intermediates, leading to improved reduction efficiency and selectivity; increased stereoselectivity favoring the syn product—the ionic medium influences transition-state stabilization, resulting in a higher proportion of the desired syn isomer, particularly evident in [BMIM]PF₆ (83:17 syn:anti ratio), compared to ethanol, where the ratio was significantly lower; and greater sustainability and safety—unlike ethanol, which is flammable, volatile, and generates hazardous waste, ionic liquids eliminate solvent evaporation, reduce environmental impact, and offer safer handling conditions in laboratory settings. Additionally, integrating ionic liquids into undergraduate laboratory experiments aligns with the Principles of Green Chemistry, offering a sustainable, high-efficiency alternative to traditional organic solvents. By adopting ionic liquids, students gain hands-on experience with modern, eco-friendly solvent systems while reinforcing concepts of stereoselectivity, solubility effects, and solvent-dependent reaction dynamics. Moreover, the ability to recycle and reuse ionic liquids further underscores their potential as viable replacements for hazardous volatile solvents in teaching and research laboratories.
3.1. Experimental Techniques
- Commercial reagents were obtained from Thermo Scientific or VWR and were used directly as supplied or purified prior to use, following established guidelines.Non-aqueous reagents were transferred under nitrogen using a syringe. Organic solutions were concentrated under reduced pressure on a Büchi rotary evaporator using a water bath or were evaporated under a sand bath. Thin-layer chromatography (TLC) was performed on Merck aluminum-backed plates coated with 0.2 mm silica gel 60-F. Visualization of the developed chromatogram was conducted using UV fluorescence quenching at 254 nm.1H and 13C NMR spectra were recorded on a Jeol JNM-ECZ (400 MHz for protons). Data for 1H NMR are reported as follows: chemical shift (δ-ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), integration, and coupling constant (Hz). Data for 13C NMR spectra are reported in terms of chemical shift (ppm) downfield from TMS.IR spectra were recorded on a Bruker Alpha spectrometer using direct ATR application. All absorptions are reported in terms of frequency of absorption (cm-1).
3.2. Experimental
- (3aR,4S,5R,6aS)-5-methyl-4-(nitromethyl)-hexahydrocyclopenta[b]furan-2-one (2)(3a,R,4S,5R,6aS)-4-(aminomethyl)-5-methyl-hexahydrocyclopenta[b]furan-2-one (3)2-(1R,2S,3R,5R)-5-hydroxy-3-methyl-2-(nitromethyl)cyclopentyl)acetic acid (4)
 Method ATo a stirred solution of [(1S,2R,3S)-3-methyl-2(nitromethyl)-5-oxocyclopentyl]acetic acid (1) (2.00 g, 9.95 mmol, 1.00 equiv) in [BMIM]PF₆ (10 mL) within an Erlenmeyer flask, sodium borohydride (NaBH₄) was added at room temperature (489 mg, 12.94 mmol, 1.30 equiv) in small portions over a period of 15 minutes. The resulting reaction mixture was stirred for an additional 30 minutes and subsequently poured into ice water (10 mL). Dilute hydrochloric acid (5%) was then added until a pH of 2 was achieved, at which point thin-layer chromatography (TLC) analysis indicated the formation of a new product. The aqueous layer was extracted with ether (4 x 10 mL), the extract was dried over magnesium sulfate (MgSO₄), and concentration under reduced pressure afforded a mixture (1.82 g, 91% yield). Column chromatography on silica, eluting with a hexane:ether mixture (5:1), yielded a mixture of compounds: (2), (3), and (4) (1.35 g, 74%; 0.22 g, 12%; 0.25 g, 14%, respectively).(2) was obtained as a colorless oil (3.9 g, 85% yield); νmax (thin film) 2968, 2931, 1769, 1550, 1381, 1172; δH (400 MHz, CDCl₃) 4.92 (1H, q, J 5.2 Hz, J 2.2 Hz, CHO), 4.60 (1H, dd, J 7.5 Hz, J 4.7 Hz, CH2NO₂), 4.37 (1H, dd, J 8.4 Hz, J 3.8 Hz, CH2NO₂), 2.78 (1H, dd, J 9.8 Hz, J 8.4 Hz, J 1.6 Hz, CH2C=O), 2.73 (1H, ddd, J 5.6 Hz, J 1.9 Hz, CHCHO), 2.54-2.47 (1H, m, CH2CHO), 2.44 (1H, dd, J 15.9 Hz, J 1.9 Hz, CH2C=O), 2.06-2.03 (1H, m, CHCH₂NO₂), 1.82-1.78 (1H, m, CHCH₃), 1.62-1.54 (1H, m, CH2CHO), 1.11 (3H, d, J 6.5 Hz, CH3); δC (100 MHz, CDCl₃) 176.2, 83.1, 77.4, 50.5, 43.5, 40.6, 37.4, 34.0, 17.4; m/z (C.I) 200 (MH⁺, 100%), 182 (54%), 153 (58%), 131 (60%), 108 (40%), 82 (21%) C₉H₁₄N₄, requires 200.0924, found 200.0916.(3) was obtained as a colorless oil (196 mg, 12% yield) with the following spectral data: υmax (thin film/cm⁻¹) 3402, 2957, 1718, 1549; δH (400 MHz, CDCl₃) 4.49 (2H, d, J 6.6 Hz, CH2NH₂), 4.04 (1H, ddd, J 5.0 Hz, J 2.9 Hz, J 2.8 Hz, CHOH), 2.52 (1H, dd, J 11.8 Hz, J 4.9, CH2C=O), 2.38 (1H, dd, J 9.7 Hz, J 7.3 Hz, J 2.3 Hz, CH2C=O), 2.11-2.03 (2H, m, CHCH₂C=O, CHCH₃), 1.93-1.83 (2H, m, CHCH₂NH₂, CH2CHO), 1.59-1.54 (1H, m, CH2CHO), 1.06 (3H, d, J 6.0 Hz, CH₃CH); δC (100 MHz, CDCl₃) 177.0, 78.7, 77.4, 49.7, 48.8, 41.1, 37.5, 35.6, 19.3; m/z (C.I) 153 (M⁺-NH₂, 96%), 139 (20%), 132 (28%), 109 (21%), 107 (51%) C₉H₁₅N₂, requires 153.0916, found 153.0912.(4) was obtained as a white powder (81 mg, 4% yield) with a melting point of 65-67°C; νmax (KBr/cm⁻¹) 3461, 2967, 2936, 1733, 1553, 1378; δH (400 MHz, CDCl₃) 4.56 (1H, dd, J 8.8 Hz, J 3.6 Hz, CH2NO₂), 4.29 (1H, dd, J 8.3 Hz, J 3.6 Hz, CH2NO₂), 3.41 (1H, m, CHOH), 2.42-2.36 (2H, m, CH2C=O), 2.01 (1H, m, CHCHOH), 1.86-1.83 (1H, m, CH2CHOH), 1.78-1.74 (1H, m, CHCH₂NO₂), 1.68-1.64 (1H, m, CHCH₃), 1.58-1.56 (1H, m, CH2CHOH), 1.08 (3H, d, J 6.4 Hz, CH3); δC (100 MHz, CDCl₃).
Method ATo a stirred solution of [(1S,2R,3S)-3-methyl-2(nitromethyl)-5-oxocyclopentyl]acetic acid (1) (2.00 g, 9.95 mmol, 1.00 equiv) in [BMIM]PF₆ (10 mL) within an Erlenmeyer flask, sodium borohydride (NaBH₄) was added at room temperature (489 mg, 12.94 mmol, 1.30 equiv) in small portions over a period of 15 minutes. The resulting reaction mixture was stirred for an additional 30 minutes and subsequently poured into ice water (10 mL). Dilute hydrochloric acid (5%) was then added until a pH of 2 was achieved, at which point thin-layer chromatography (TLC) analysis indicated the formation of a new product. The aqueous layer was extracted with ether (4 x 10 mL), the extract was dried over magnesium sulfate (MgSO₄), and concentration under reduced pressure afforded a mixture (1.82 g, 91% yield). Column chromatography on silica, eluting with a hexane:ether mixture (5:1), yielded a mixture of compounds: (2), (3), and (4) (1.35 g, 74%; 0.22 g, 12%; 0.25 g, 14%, respectively).(2) was obtained as a colorless oil (3.9 g, 85% yield); νmax (thin film) 2968, 2931, 1769, 1550, 1381, 1172; δH (400 MHz, CDCl₃) 4.92 (1H, q, J 5.2 Hz, J 2.2 Hz, CHO), 4.60 (1H, dd, J 7.5 Hz, J 4.7 Hz, CH2NO₂), 4.37 (1H, dd, J 8.4 Hz, J 3.8 Hz, CH2NO₂), 2.78 (1H, dd, J 9.8 Hz, J 8.4 Hz, J 1.6 Hz, CH2C=O), 2.73 (1H, ddd, J 5.6 Hz, J 1.9 Hz, CHCHO), 2.54-2.47 (1H, m, CH2CHO), 2.44 (1H, dd, J 15.9 Hz, J 1.9 Hz, CH2C=O), 2.06-2.03 (1H, m, CHCH₂NO₂), 1.82-1.78 (1H, m, CHCH₃), 1.62-1.54 (1H, m, CH2CHO), 1.11 (3H, d, J 6.5 Hz, CH3); δC (100 MHz, CDCl₃) 176.2, 83.1, 77.4, 50.5, 43.5, 40.6, 37.4, 34.0, 17.4; m/z (C.I) 200 (MH⁺, 100%), 182 (54%), 153 (58%), 131 (60%), 108 (40%), 82 (21%) C₉H₁₄N₄, requires 200.0924, found 200.0916.(3) was obtained as a colorless oil (196 mg, 12% yield) with the following spectral data: υmax (thin film/cm⁻¹) 3402, 2957, 1718, 1549; δH (400 MHz, CDCl₃) 4.49 (2H, d, J 6.6 Hz, CH2NH₂), 4.04 (1H, ddd, J 5.0 Hz, J 2.9 Hz, J 2.8 Hz, CHOH), 2.52 (1H, dd, J 11.8 Hz, J 4.9, CH2C=O), 2.38 (1H, dd, J 9.7 Hz, J 7.3 Hz, J 2.3 Hz, CH2C=O), 2.11-2.03 (2H, m, CHCH₂C=O, CHCH₃), 1.93-1.83 (2H, m, CHCH₂NH₂, CH2CHO), 1.59-1.54 (1H, m, CH2CHO), 1.06 (3H, d, J 6.0 Hz, CH₃CH); δC (100 MHz, CDCl₃) 177.0, 78.7, 77.4, 49.7, 48.8, 41.1, 37.5, 35.6, 19.3; m/z (C.I) 153 (M⁺-NH₂, 96%), 139 (20%), 132 (28%), 109 (21%), 107 (51%) C₉H₁₅N₂, requires 153.0916, found 153.0912.(4) was obtained as a white powder (81 mg, 4% yield) with a melting point of 65-67°C; νmax (KBr/cm⁻¹) 3461, 2967, 2936, 1733, 1553, 1378; δH (400 MHz, CDCl₃) 4.56 (1H, dd, J 8.8 Hz, J 3.6 Hz, CH2NO₂), 4.29 (1H, dd, J 8.3 Hz, J 3.6 Hz, CH2NO₂), 3.41 (1H, m, CHOH), 2.42-2.36 (2H, m, CH2C=O), 2.01 (1H, m, CHCHOH), 1.86-1.83 (1H, m, CH2CHOH), 1.78-1.74 (1H, m, CHCH₂NO₂), 1.68-1.64 (1H, m, CHCH₃), 1.58-1.56 (1H, m, CH2CHOH), 1.08 (3H, d, J 6.4 Hz, CH3); δC (100 MHz, CDCl₃). Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML