-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2024; 12(1): 20-24
doi:10.5923/j.ajoc.20241201.02
Received: Mar. 20, 2024; Accepted: Apr. 7, 2024; Published: Apr. 13, 2024

Chemical Investigation on the Chloroform Fraction of the Aerial Parts of Leucas zeylanica
Uttam Chowdhury1, Kazal Baran Nath2, Ranjit Kumar Sutradhar3, Sreebash Chandra Bhattarcharjee4
1Additional Chief Chemist, Chittagong Urea Fertilizer Limited, Bangladesh
2Associate Professor, Institute of Education & Research (IER), University of Chittagong, Bangladesh
3Professor, Department of Chemistry, Chittagong University of Engineering &Technology, Chittagong, Bangladesh
4Principal Scientific Officer, Bangladesh Council of Scientific and Industrial Research (BCSIR), Chattogram
Correspondence to: Kazal Baran Nath, Associate Professor, Institute of Education & Research (IER), University of Chittagong, Bangladesh.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Chemical Investigation on the Chloroform Fraction of the Aerial Parts of Leucas zeylanica were studied. The plant materials were clipping into small pieces and dried in air absence of sunlight. The dried materials were grinded into powder in a Cyclotech Machine (200 mesh) and stored in an air tight container. The air dried powder 5 kg was successively extracted with n-hexane (3×72 h), chloroform (3×72 h) and MeOH (3×72 h) respectively. On removal of the solvent, the n-hexane extract gave a light green Mass (18.5 gm), chloroform extract gave a deep green Mass (43 gm). and MeOH extract gave a light yellow mass (55g). The extracts, were concentrated to dryness under controlled temperature 45°C. Fractionation and purification of chloroform extract by chromatographic method afforded the new compound (A). The compound was characterized by chemical and spectroscopic method.
Keywords: TLC, PTLC, IR, NMR, MS
Cite this paper: Uttam Chowdhury, Kazal Baran Nath, Ranjit Kumar Sutradhar, Sreebash Chandra Bhattarcharjee, Chemical Investigation on the Chloroform Fraction of the Aerial Parts of Leucas zeylanica, American Journal of Organic Chemistry, Vol. 12 No. 1, 2024, pp. 20-24. doi: 10.5923/j.ajoc.20241201.02.
1. Introduction
- The genus Leucas (Lamiaceae) comprises about 80 species [Hedge, 1990]. The highest species diversity has been found in East Africa [Ryding, 1998]. In India, 43 species are available [Murkerjee, 1940]. The previous taxonomic accounts of Leucas for the present Bangladesh area have been given by Hooker (1885), Prain (1903) and Kangilal et al. (1939) who reported only five species. But eight species of the genus Leucas R. Br. (Lamiaceae) have been recognized for Bangladesh are given below [Mahbuba Khanam et al., 2005]. 1) Leucas aspera, 2) Leucas biflora, 3) Leucas cephalotes, 4) Leucas ciliata, 5) Leucas indica, 6) Leucas mollissima, 7) Leucas vestita and 8) Leucas zeylanica. Leucas zeylanica is one of the important medicinal plant of Bangladesh. The Bengali name of the plant is Kusha or Shetadrone. The tribal name of the plant is Pai Thung Sa (Marma). It belongs to the Lamiaceae family. Its English name is slitwort. It is a small, terrestrial, herbaceous, annual erect plant or sometimes tufted, hispid and aromatic plant growing to a height of upto 45 cm, stipules absent. Stem is green in color; It is qudra angular plant. Leaves are elliptic in shape and green in color which occur opposite sides of stemps and large in number. These are subsessile leaves which are linear lanceolate or elliptic lanceolate which is 2.5 to 7.5 cm long. Not lobed or divided, blunt at the tip, obtuse entire or cerrulate, gradular hispid and coarsely dentate at the margin. Roots are mainly tap root and fibrous which is white or brown in color. It is cultivated in home gardens for use in local medicine and as a pot herb. It is widely distributed throughout Southeast Asia is consisting of the countries that are south of China and Japan, east of India, west of Papua New Guinea and north of Australia but is rather rare in East Asia. Juice of the herb is used in headache, colds, scabies and other skin diseases. Decoction of leaves is used as a lotion for ulcers of the nose. The leaves are anthelmintic, diaphoretic, stimulant and Kulnerary. They are applied topically to heal wounds. The leaves are used as a poultice to treat itch and vertigo. The entire plant is externally rubbed on abdomen after childbirth in human pregnant [Harborne J.B. et al., 1999]. The sap of the leaves is used for sores of eyes and nostrils. Also it is used as a vermifuge with children. The plant is used medicinally for coughs, toothaches and abdominal pains. In Malaysia, the leaves may be taken as a sedative and to teal wounds. The whole herb has a bitter taste, but is still used as a pot herb. This was the first report that Leucas zeylanica showed antifungal activity against dermatophytes [Babu et al., 2016]. The microbs such as E.Coli and Coliforms growth were inhibit when exposed to leaves extract of Leucas zeylanica [Zhang et al., 2016]. In China and Malaya poultice of leaves is used for wounds and sores. In Srilanka it is used for anorexia, flatulence, colic, in mixture, used to treat malaria. In Thailand leaves, roots and flowers are used for weaning. Poultice of leaves used for wound healing and to stop bleeding. Najat Nidhal et al. (2020) studied on Leucas zeylanica. In this study, thirty compounds were isolated from Leucas zeylanica, including two norditerpenoid, three flavonoid glycosides, six flavonoids, two phytosterols, two phenylpropanoids, five terpenoids, one aliphatic glycoside, one nucleobase, one amino acid, two alkaloids and one cytochalasin. Previous phytochemical screening on the genus Leucas zeylanica indicated that very little phytochemical study on Leucas zeylanica was carried out. Therefore, it demands more phytochemical investigations. Research project on the aerial parts of Leucas zeylanica has been undertaken to achieve the following objectives: 1. The present study has been undertaken for detailed investigation of the aerial parts of Leucas zeylanica with an aim for isolation, purification and structural elucidation of the various secondary metabolites. 2. Our principal objective was to isolate and purify steroid and terpenoid along with other constituents from the aerial parts of Leucas zeylanica as well as to determine the molecular structure of the isolated secondary metabolites by various physical and chemical methods.
2. Methods & Materials
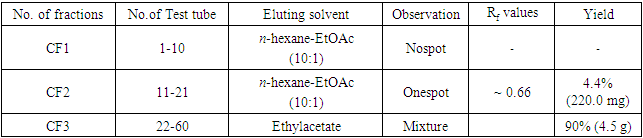
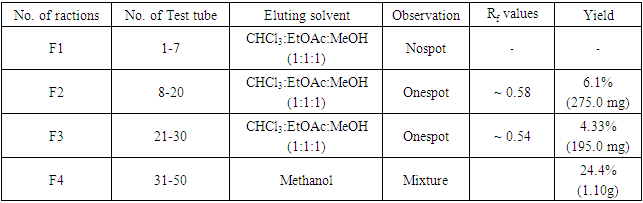
- The aerial parts of Leucas zeylanica were collected from the campus area of Chittagong University of Engineering & Technology. For the experimental purpose only the matured and fresh plants were collected during the month of December 2018.The plants were chopped into small pieces and dried in air in absence of sunlight. The dried materials were grinded into powder in a cyclotech-grinding machine (200 mesh). The powder was stored in polythene packets until used for extraction.Solvents and Chemicals All the solvents and chemicals used in the extractions and experiments were procured from E. Merk(Germany) or BDH (England) or Aldrich (America) and were either meant for laboratory use or were of analytical reagent grade. All solvents, like DCM chloroform, ethyl acetate, methanol and rectified spirit were distilled prior to use for extraction, chromatographic separation or any analytical purpose.Extraction and fractionation of Leucas zeylanica The air-dried powder (5kg) of Leucas zeylanica was successively extracted with n- hexane (3×72 h), CHCl3 (3×72 h) and MeOH (3×72 h) respectively as described in the fractionation Scheme 1. On removal of the solvent, the n-hexane extract gave a light green Mass HE (18.5g), CHCl3 extract gave a deep green Mass CE (43.0 g) and the MeOH extract gave a light yellow Mass ME (55.0 g).
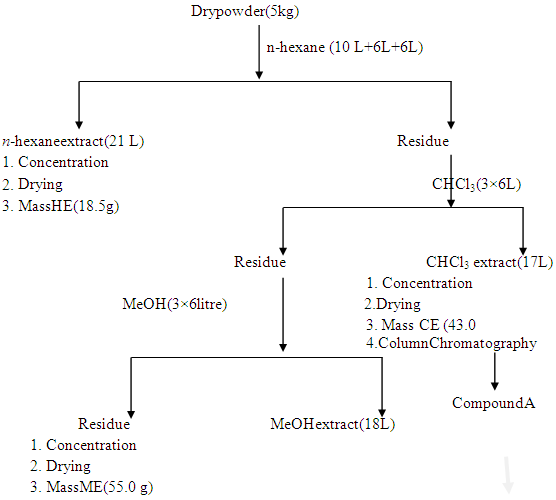 | Scheme 1. Extraction and fractionation of plant materials |
|
|
3. Results & Discussion
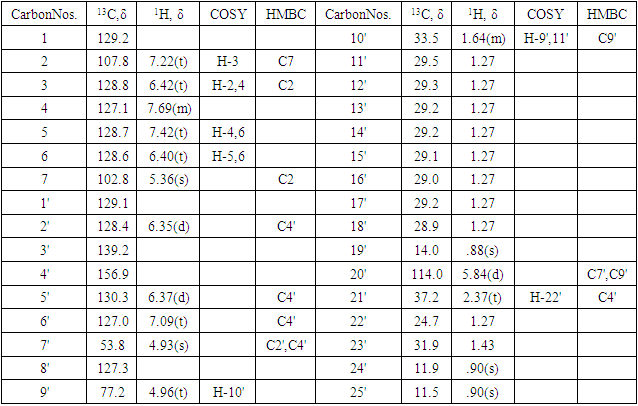
- Compound A (180 mg) was an amorphous solid, m.p 170-172°C. Compound A did not give Salkowski and Liebermann-Burchard reaction for steroid and terpenoid. The IR spectrum of A showed broad absorption for O-H group at νmax 3380 cm-1 and a weak absorption at Vmax1603 cm-1 for C=C function in the molecule. The 13C NMR spectrum of A showed the presence of 32 carbons in the molecule (Table 3). The mass spectrum of A exhibiteda molecular ion peak at m/z:481.5632[M+1] consistent with the molecular formula C32H48O3. The 13C DEPT spectrum of A revealed the presence of 13 methylene carbons, 11methine carbons and 3 methylcarbons in the molecule. The absorption positions of all 32 carbons in 13C NMR spectrum are given in Table 3. The 1H NMR spectrum of A showed the presence of 8 aromatic protons attached to C2(7.22t), C3(6.42t), C4(7.69m), C5(7.42t), C6(6.40t), C2'(6.35d), C5'(6.37d), and C6'(7.09t). The absorption positions of two olefinic proton sattached to C20'(5.84d), 3 methine protons attached to C7(5.36s), C9'(4.96t) and C23'(1.43) are shown in 1H NMR spectrum of A. Absorption positions of 12 sets of methylene protons and 3 sets of methyl protons are given in the Table 3. Absorption patterns, number of carbons and protons of compound A suggest the presence of two side chains in the molecule. The molecular ion peak of compound A m/z:481.5632 [M+1] along with other mass peaks m/z: [M+1] 463, 410, 340, 284, 218, 182,162, 123 etc can be explain on the given structure of A. The important H-H correlations in 2D COSY spectrum and H-C correlations in HMBC spectrum are presented in Fig-1 and Table- 3. Thus on the basis of IR,1H NMR, 13C NMR and mass spectra, structure 1 was confirmed to the compound A. Compound A is thus characterized as 4'-[7-[hydroxyl (phenyl) methoxy]- 3'-isopentyl benzyl] tridec-8'-en-9'-ol and is reported first time from Leucas zeylanica.
|
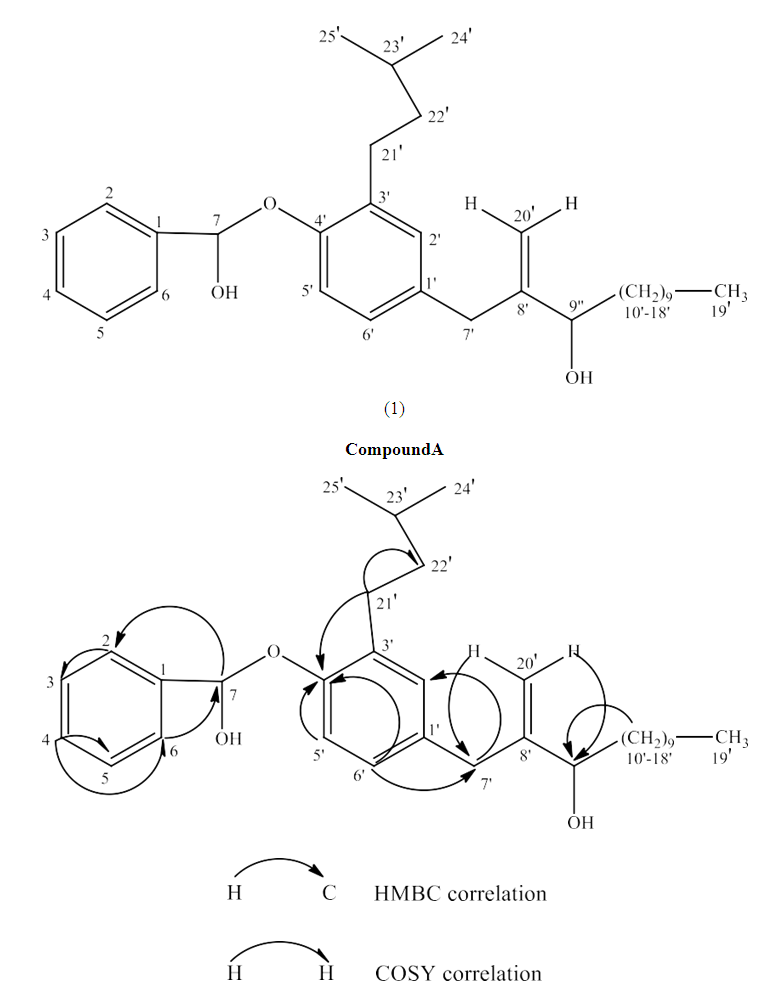 | Figure 1. Important COSYand HMBC correlations of compound A |
4. Conclusions
- The principal objective of this research work was to isolate biologically active compounds along with other secondary metabolites from the aerial parts of Leucas zeylanica as well as to determine the molecular structure of the isolated compounds. We are successful to finding out some of them. We are able to isolate and establish the structure of the compound A. The structure determination of the mentioned compound from Leucas zeylanica was first time reported from the plant body.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML