-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2019; 9(2): 25-27
doi:10.5923/j.ajoc.20190902.01

Comparison of Various Feedstocks for the Microwave-Assisted Synthesis of Biodiesel
C. Villot, M. E. Howard, K. W. Kittredge
Department of Chemistry, Virginia Wesleyan University, 5817 Wesleyan Dr., Virginia Beach, VA
Correspondence to: K. W. Kittredge, Department of Chemistry, Virginia Wesleyan University, 5817 Wesleyan Dr., Virginia Beach, VA.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The microwave-assisted acid catalyzed synthesis of biodiesel from various feedstocks are reported. The feedstocks consist of vegetable, canola, olive, avocado, corn, cottonseed and soybean oils and also were compared to a commercial biodiesel sample that was prepared from a mixture of varying oils by GC-MS. All oils gave biodiesel in excellent yields (<85%). Combustion analysis gave heats of combustion between 40.75 to 43.45 kJ/g, similar to literature values.
Keywords: Microwave-Assisted Synthesis, Biodiesel
Cite this paper: C. Villot, M. E. Howard, K. W. Kittredge, Comparison of Various Feedstocks for the Microwave-Assisted Synthesis of Biodiesel, American Journal of Organic Chemistry, Vol. 9 No. 2, 2019, pp. 25-27. doi: 10.5923/j.ajoc.20190902.01.
Article Outline
1. Introduction
- The rapid consumption of petroleum fuels and their increasing prices have come to worldwide attention. Due to their future environmental and economic impacts. [1] This worldwide ecological and economical movement has resulted in the search for alternative fuel sources. [2] Biodiesel is one potential fuel aimed at reducing the usage of petroleum fuels. [3] Unlike petroleum fuels, biodiesel fuels can be nontoxic and biodegradable. [2]. Their production and consumption is a closed carbon cycle and CO2 emissions may be reduced by as much as 78%. [4-5] Biodiesel is commonly produced from waste vegetable oil and consists of fatty acid esters of various lengths (Figure 1), however other oils may be substituted in place of vegetable oil. [68]
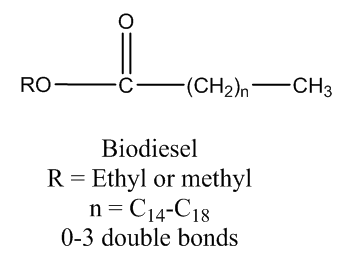 | Figure 1. Structure of biodiesel |
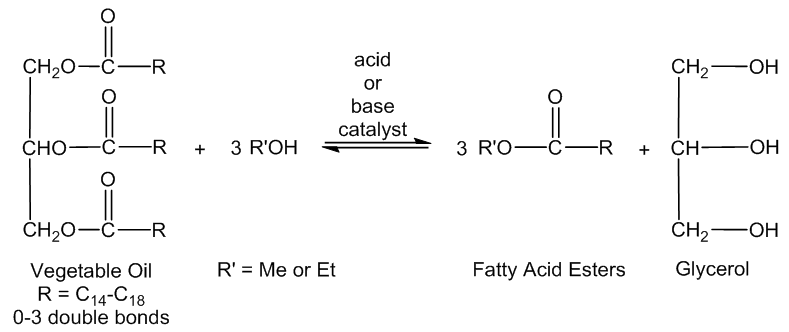 | Figure 2. Reaction scheme for the synthesis of biodiesel from vegetable oil |
2. Materials and Methods
- Materials and EquipmentAll transesterification trials were carried out using p-toluenesulfonic acid (Fisher) and methanol (Fisher) and were used as received. A CEM Mars 6 microwave reactor was used for the microwave-assisted reactions. The synthesized biodiesel product was identified and characterized by GC-MS using standard literature methods. [12-15] Percent conversions were determined by GC-MS. [15] The GC-MS was an Agilent 5977A Extractor XL MSD with a chemical ionization detector. Split injections with a 100:1 split ratio. The GC was fitted with an HP-5 (5% phenyl: 95% methyl silicone) column (dimensions: 30 m x 0.32 mm; flow rate:1.64 mL/min; Injector temperature: 250°C; Oven temperature: 150°C; Detector temperature: 290°C). A Parr Instruments 1341 Oxygen Bomb Calorimeter with an 1108 Oxygen Combustion Bomb was used to measure the heats of combustion for the synthesized biodiesel and the commercially available petroleum diesel. Temperature changes were measured and recorded using a stainless steel temperature probe connected to a Vernier LabPro interface running LoggerPro v3.10.1.General ProceduresReaction Solutions: All reactions were done in a 200 mL round bottom flask equipped with a teflon coated magnetic stir bar and a reflux condenser. The solution was refluxed by setting the heating temperature to 65°C for 1 h. Each reaction solution was prepared using 15 mL oil, 5 g (30 mmol) of p-toluenesulfonic acid, and 25 mL of methanol.Phase Separation of the Biodiesel and GlycerolAfter completion of the reaction, the mixture was allowed to cool, gravity filtered through filter paper to remove the acid catalyst and separated into two layers. The top layer, lighter in color, was the biodiesel; and the bottom layer, darker in color, was the glycerol. The biodiesel layer, which also contained some unreacted methanol, was collected and the methanol was removed in vacuo by rotary evaporation. Purification of the BiodieselInto the biodiesel fraction were added 25 g of magnesium sulfate and 100 mL of methanol. The suspension was swirled occasionally and then cooled on ice for an additional 5 minutes. The solid magnesium sulfate was removed by vacuum filtration and the methanol was removed in vacuo by rotary evaporation. The biodiesel was used as is in the combustion analysis.Heats of CombustionThe temperature was recorded during the calorimetry runs as two readings per second over a typical 1000 second run. After calibration of the stainless steel temperature probe and calibration of the bomb calorimeter with benzoic acid, 1.0 g samples of synthesized biodiesel fuel (or commercially purchased diesel fuel) were combusted. A minimum of five trials for each sample were done.
3. Results and Discussion
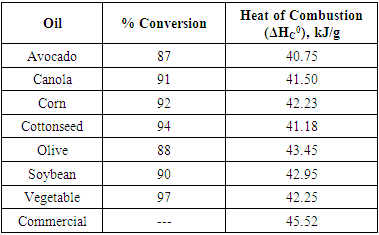
- Bomb calorimetry was used to quantify the difference in quality of the biodiesel products and as well as compare to a commercially available petroleum diesel sample. Calorimetric combustion studies of biodiesel were found to be between 40.75 to 43.45 kJ/g (Table 1) and are similar to those reported in the literature. [12] These values are 5-10% lower than the heat of combustion for a commercial petroleum diesel sample (45.52 kJ/g). All oils gave a high % conversion, the lowest being for avocado oil (87%) and the highest for vegetable oil (97%).
|
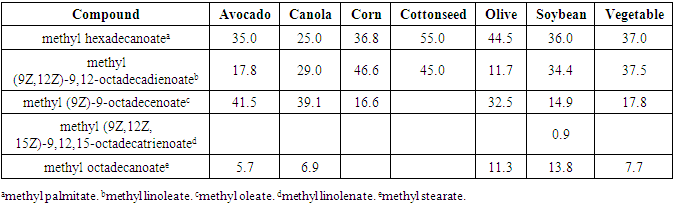
|
4. Conclusions
- We report the acid catalyzed microwave-assisted synthesis of biodiesel from various oils. We found no significant difference in the combustion analysis between the oils, but all were lower than that for commercial diesel. In the chemical composition of the different biodiesel products showed that all the oils contained the same three major components, with the exception of cottonseed oil. The source of oil for the production of biodiesel is shown to be wide ranging and not limited to exclusively vegetable oil.
ACKNOWLEDGEMENTS
- This work was supported by the National Science Foundation (CHE-1429247).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML