-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2019; 9(1): 14-24
doi:10.5923/j.ajoc.20190901.03

Experimental and Computational Study of Antioxidant Activities of Synthetic Heterocyclic Quinazoline-4-one Derivatives
Nagwa M. M. Hamada1, Alshimaa Abd Elgawad2
1Department of Chemistry, Faculty of Education, Alexandria University, Alexandria, Egypt
2Department of Biochemistry, Faculty of Science, Alexandria University, Alexandria, Egypt
Correspondence to: Nagwa M. M. Hamada, Department of Chemistry, Faculty of Education, Alexandria University, Alexandria, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The objective of this study was to investigate and evaluate both vanillin and quinazolinone ring systems as anti-radical agents. Herein, we discuss the design strategy and report the synthesis of five novel antioxidants as quinazolinone–vanillin derivatives. The structures of the synthesized heterocyclic compounds have been recognized based on their melting point (m.p.), elemental analysis, IR and 1H & 13C NMR and mass spectroscopic data. In this work, we report a combined experimental and theoretical study of the radical scavenging activity of the synthesized heterocyclic compounds to determine their potential as antioxidants. The optimization of all the synthesized compounds using density functional theory (DFT) at the B3LYP/6-311G (d, p) & 631++ level of theory was established. Radical scavenging activity has been explained based on the energy gap (HOMO–LUMO) and ionization potential (IP) values. The calculations carried out are related to the electronic affinity (EA), hardness (η), softness (S), electronegativity (χ), and electrophilicity (ω). The structure activity relationship has been explained by mapping the electrostatic potential surface (MEP). The results of both experimental and theoretical antioxidant activities of the synthesized molecules have shown that the synthesized compounds (2–4) demonstrated simulated results comparable with those of vanillin, they are good antioxidants and could be effective in fighting oxidative attacks. The results revealed also that the synthesized compounds (5 and 6) are more effective antioxidants than common vanillin and they showed excellent scavenging capacity against DPPH and Nitric oxide (NO).
Keywords: Antioxidant activity, DPPH, Density functional theory, Molecular properties, Ionization potential, MEP
Cite this paper: Nagwa M. M. Hamada, Alshimaa Abd Elgawad, Experimental and Computational Study of Antioxidant Activities of Synthetic Heterocyclic Quinazoline-4-one Derivatives, American Journal of Organic Chemistry, Vol. 9 No. 1, 2019, pp. 14-24. doi: 10.5923/j.ajoc.20190901.03.
Article Outline
1. Introduction
- A variety of aza-heterocycles have been proven to be very effective in various therapeutic diseases due to their wide and distinct biopharmaceutical activities. Quinazolin-4(3H)-ones [1-5] are classes of fused heterocycles that are of significant interest because of their biological activities such as anti-cancer [6,7], antitumor [8-12], antioxidant [13], antimicrobial [14,15], antifungal [16], anticonvulsant [17,18], anti-HIV [19], anti-HCV [20] anti-TMV [21] activities. Recently, a methodological study of the literature suggested that this type of fused heterocycles has a broad spectrum of applications as antioxidants [22-28]. Furthermore, there are many different reports that evaluate the antioxidant activity of vanillin against different free radicals [29-33]. On the other hand, quantum chemical calculations and density functional theory (DFT) were applied to calculate the ionization potential (IP) and other radical scavenging properties of antioxidant systems [34-41]. All the above observations provide motivation to develop an appropriate strategy for designing a series of compounds containing quinazolin-4(3H)-one ring systems associated with a vanillin moiety to evaluate their antioxidant activity experimentally and via computational calculations using DFT. The theoretical results were compared with experimental data and were found to be in good agreement.
2. Results and Discussions
2.1. Chemistry
- The synthesis of quinazolinone derivatives (Scheme 1) was carried out using 2-hydrazino-4- quinazolinone (2), which was obtained previously, from the reaction of 2-mercapto -3- phenyl-2,3-dihydro-1H-quinazolin-4-one (1) with hydrazine hydrate [42,43].
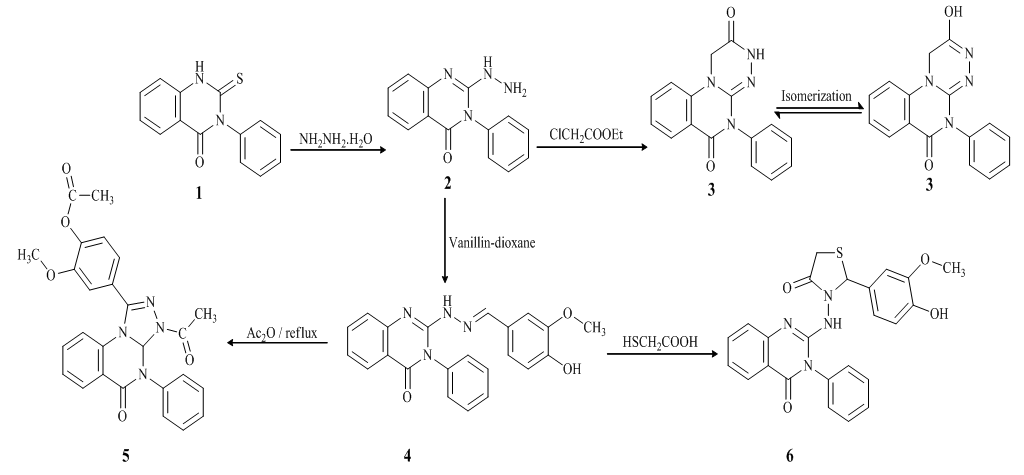 | Scheme 1. Synthetic pathways for the studied compounds (2–6) |
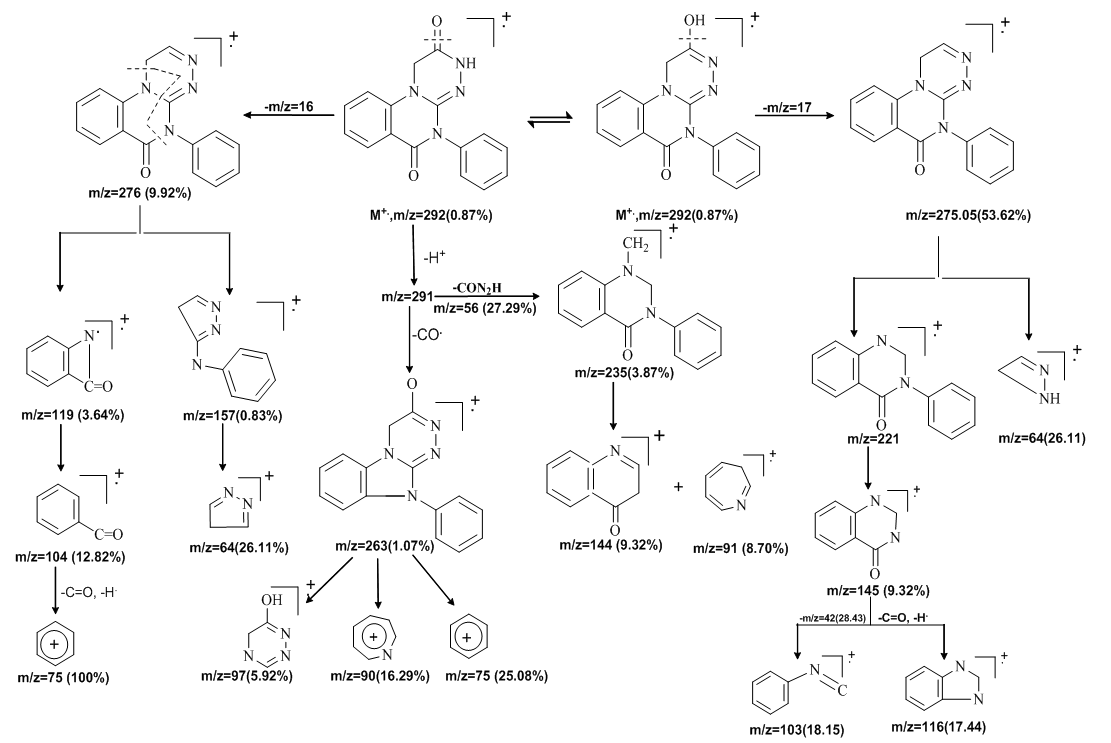 | Scheme 2. Fragmentation pattern for the mass spectrum of compounds (3) |
2.2. Estimation of Antioxidant and Anti-Inflammatory Activities
- Heteroaromatic substitution is an attractive strategy for the development of drugs with desirable activities and several reports on the pharmacological activity of heterocyclic vanillin derivatives provided with the motivation to synthesize such analogs and estimate their antioxidant and antiinflammatory activities. Thus, we have designed our study for the synthesis and characterization of quinazoline-4-one derivatives to be evaluated for their antioxidant and antiinflammatory activities. Further identification based on computational studies using density functional theory (DFT) was carried out.
2.2.1. Examination of Antioxidant Activity Using a DPPH Scavenging Assay
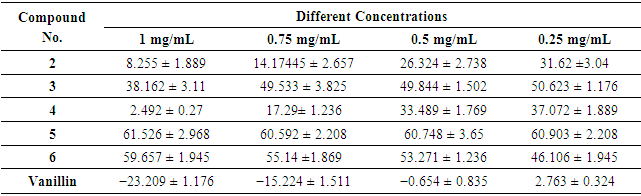
- DPPH radical scavenging is an extensively used method to evaluate antioxidant activities. In the present study, the antioxidant activity of the synthesized compounds was measured using a freshly prepared solution of DPPH (diphenyl picryl hydrazyl) in DMF with an absorbance of around 515–520 nm, in its oxidized form and able to accept an electron or hydrogen radical to form a stable molecule. A change in color from purple to yellow indicates a decrease in the absorbance of the DPPH radical, which demonstrates potent antioxidant activity [44-48]. The results showed that the antioxidant activity of the synthesized compounds is attributed to the different heterocyclic rings fused with the parent quinazolin-4-one ring. Most of the tested compounds showed high to moderate interactions with the DPPH radical at different concentrations (Table 1); the antioxidant activity of these compounds is due to their electron delocalization or hydrogen radical donating ability to the DPPH radical. Compound (5) showed the highest DPPH scavenging activity with 61.526 ± 2.968 percent inhibition at a concentration of 1 mg/mL, when compared with the other compounds, which displayed more than 50% inhibition due to the presence of three e-donating groups (-OCH3, -OCOCH3 and -NCOCH3) in heterocyclic derivative (5).
|
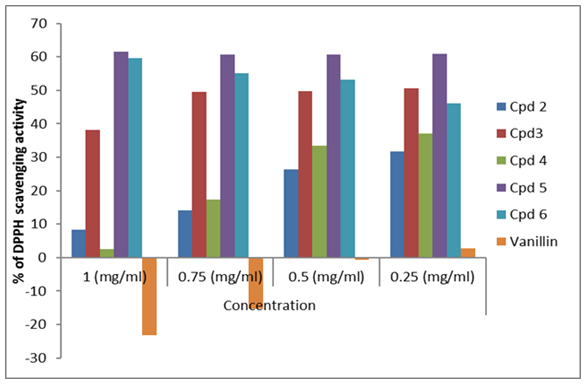 | Figure 1. Antioxidant activities of compounds using DPPH |
2.2.2. Examination of Antioxidant Activity Using Nitric Oxide (NO) Scavenging Assay
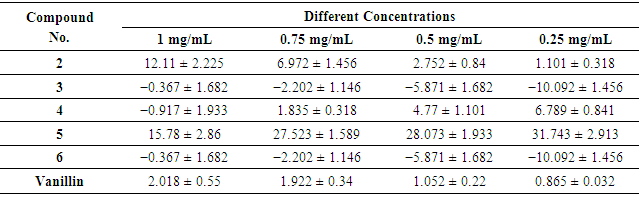
- The antioxidant properties of the synthesized compounds were examined using the nitric oxide scavenging activity assay (Figure 2). Nitric oxide (NO) is an active molecule that, under normal conditions, predominantly it has an antiinflammatory effect, also in some cases nitric oxide is considered as being a pro-inflammatory moderator that promotes inflammation [50]. The study showed that the compounds are potent nitric oxide scavengers (Table 2). In agreement with our results, Niazi et al. [34] reported that vanillin has an antiinflammatory activity that is directly proportional to its concentration and the tested compounds displayed nitric oxide radical scavenging activities in the following order: 5 > 4 > 2 > vanillin > 3 ≈ 6.
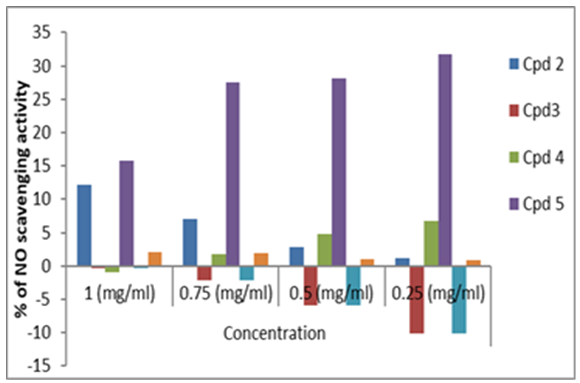 | Figure 2. Antioxidant activities of compounds using NO |
|
2.3. Computational Studies
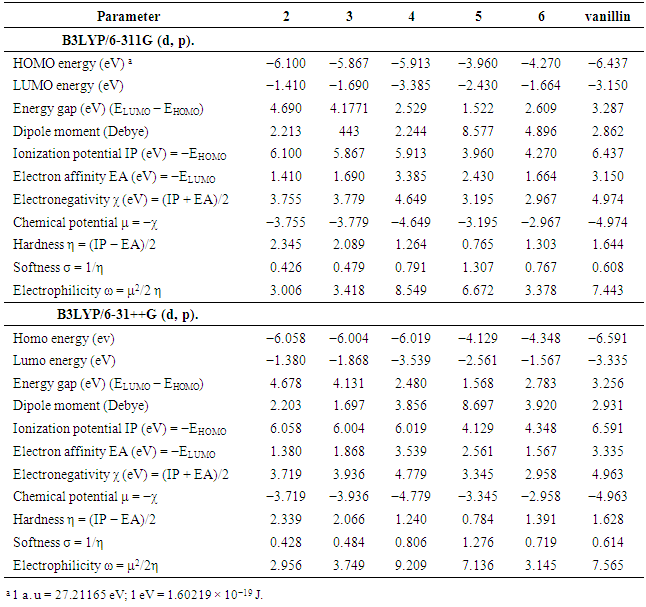
- All computational work was performed based on density functional theory (DFT) calculations [51] at the level of the B3LYP functional and 6311G & 6-31++G basis sets. The model structures of our investigated compounds (2–6) and vanillin were first designed and then optimized to obtain the minimum energy stabilized structures (Figure 3). Frontier orbitals (HOMO and LUMO) are the main participants in electronic transitions, and their energy gap depicts the reactivity [52]. Analysis of the frontier molecular orbitals by computational methods is an elegant way to explain the reactivity and electronic transitions within molecules [53].The frontier molecular orbitals (HOMOs/LUMOs along with corresponding energies) of compounds (2–6) and vanillin were explored at the B3LYP/6-311G (d,p) and B3LYP/6-31++G (d, p) levels of DFT. Energy gaps for the highest occupied and the lowest unoccupied molecular orbitals were calculated using the equation, Egap = ELUMO- EHOMO. The frontier orbitals (HOMO and LUMO) of vanillin and compounds (2–6) at the B3LYP/6-311G (d, p) level are shown in Figures 4 and 5, respectively. The π-cloud in the LUMOs of all the compounds is distributed on the quinazoline rings, except for compound (6), where the π-cloud in its LUMO orbital is distributed on the thiazolidine ring and the vanillin moiety. The π-cloud in the HOMOs of compounds (2–4) is distributed on the entire skeleton of all the C-rings, whereas the π-cloud in the HOMOs of compound (5) is distributed on the acetylated phenolic vanillin ring and the π-cloud in the HOMOs of (6) is distributed on the quinazoline ring. This explains and confirms the experimental results that indicate compounds (5,6) are good antioxidants, whereas compounds (2–4) can serve as either antioxidants or pro-oxidants. Among all the compounds, the diacetyl derivative (5) showed the lowest energy gaps, i.e., 1.522 eV calculated at B3LYP/6-311G (d, p) and 1.568 eV at B3LYP/6-31++G (d, p), while (2) showed the largest energy gaps 4.690 and 4.678 eV, respectively.For further investigation, excellent tools to describe the reactivity and stability of compounds [54-58] included selective parameters such as the ionization potential (IP), electron affinity (EA), electronegativity (χ), electronic chemical potential (μ), chemical hardness (η), softness (S), electrophilicity (ω), were calculated at B3LYP/6-311G (d, p) & B3LYP/6-31++G (d, p). The results showed that compound (5) has the lowest value of chemical hardness (η), i.e., 0.765, calculated at B3LYP/6-311G (d, p) and 0.784 eV at B3LYP/6-31++G (d, p), whereas (2) has the highest values 2.345 and 2.339 eV for both basis sets 6311 & 631++, respectively.
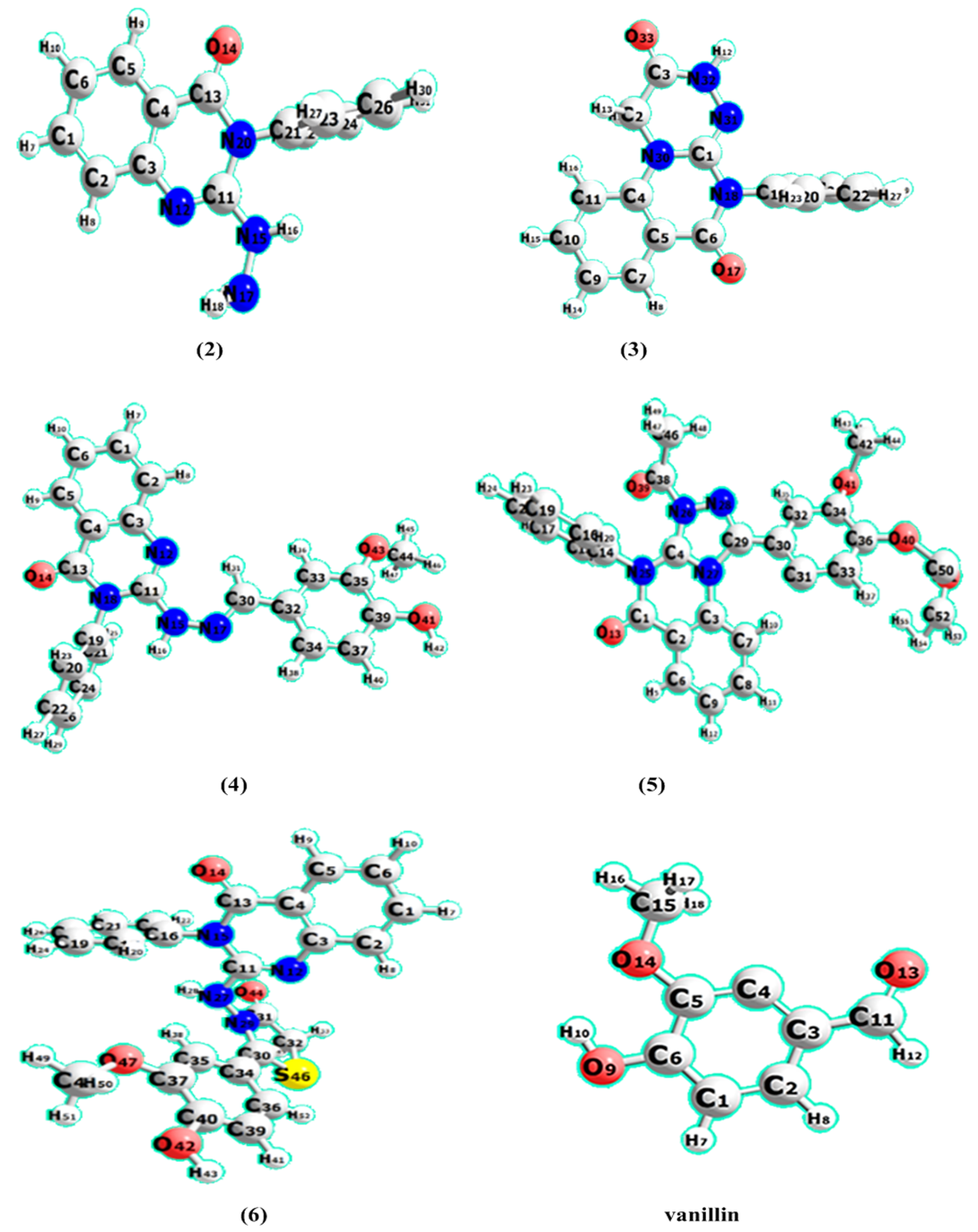 | Figure 3. The optimized geometries of compounds (2–6) and vanillin at the B3LYP/6311G (d, p) |
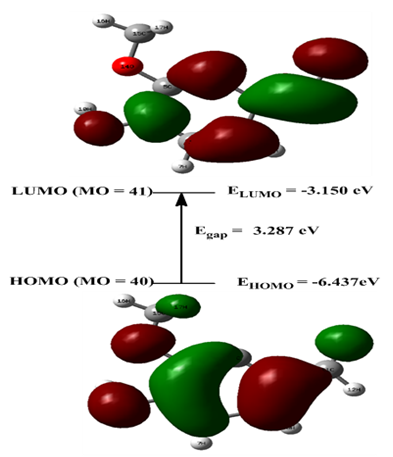 | Figure 4. HOMO and LUMO orbitals of vanillin calculated at the B3LYP/6-311G (d, p) |
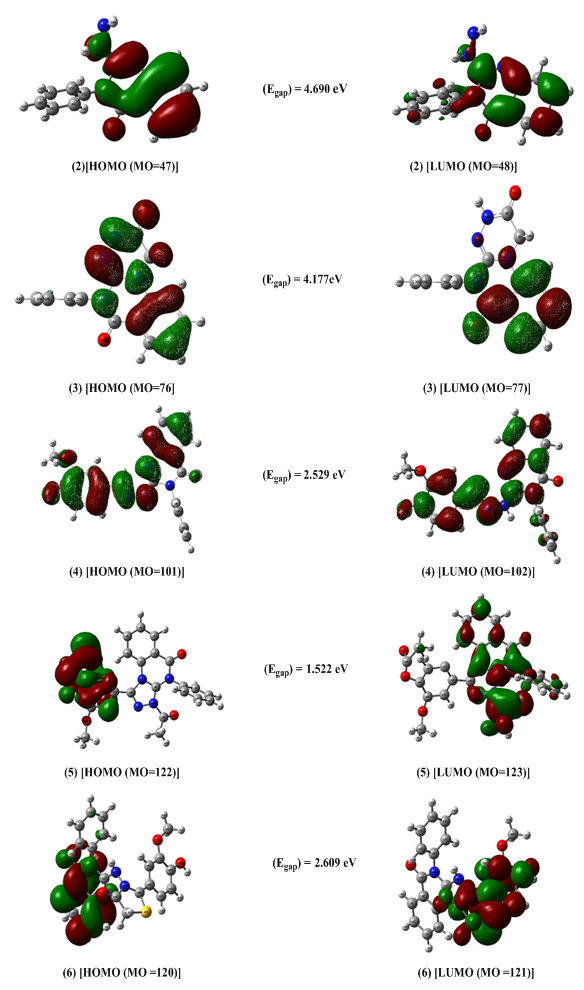 | Figure 5. HOMO and LUMO orbitals of compounds (2–6) calculated at the B3LYP/6-311G (d, p) |
|
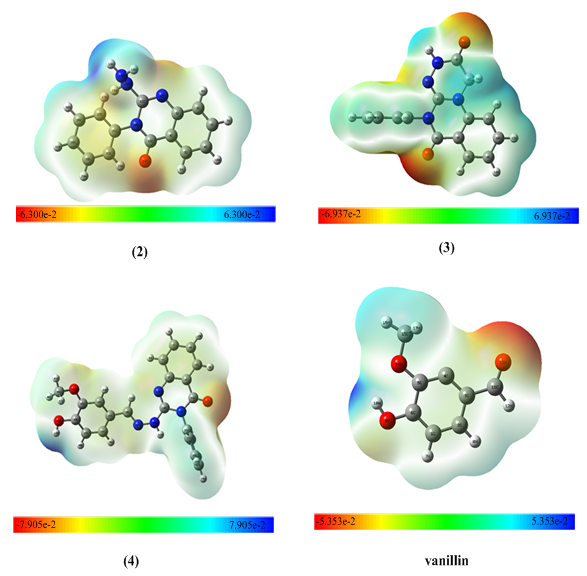 | Figure 6. Mapping the electrostatic potential surface (MEP) surface diagram of the molecules (2–4) and vanillin |
3. Experimental
3.1. General Methods
- Melting points were carried out on a Tottoli (Büchi) apparatus and were not corrected. IR (KBr) were recorded on a PerkinElmer 580 VB spectrophotometer and on a Camica 250 Hz spectrometer. Microanalyses were performed in micro analytical units, Department of Chemistry, Faculty of Science, Cairo University, Cairo, Egypt. Mass spectra were carried out on Direct Inlet part to mass analyzer on a Thermo Scientific GCMS model ISQ (70 eV El mode), performed at the Regional Center for Mycology and Biotechnology Al-Azhar University, Cairo, Egypt. The nuclear magnetic resonance spectra (1H-NMR & 13C-NMR) for the purified sample in (CDCl3) or (DMSO d6) were obtained using a Joel EAC 500 MHz FT-NMR spectrophotometer (Faculty of Science, Alexandria University, Egypt) and on a Varian Gemini VX-300 MHz spectrophotometer using TMS as an internal standard, (Faculty of Science, Cairo University, Egypt) at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Nasr city, Cairo, Egypt. The reaction progress was monitored by thin-layer chromatography (TLC) on silica gel 60 f 254 plates.
3.2. Synthesis of the Studied Compounds
- 2-Hydrazino-3-phenyl-3H-quinazolin-4-one (2)A mixture of 1 (4 g, 15.7 mmol) and hydrazine hydrate (1 g, 20 mmol) was heated under reflux in 30 mL of 95% ethanol for 5 h. Then, the reaction mixture was concentrated and poured onto crushed ice. The separated crude solid was filtered off, washed successively with water, dried and recrystallized from ethanol to give (2) as colorless needles. The yield of 2 was 77%, m.p. 200–202°C; IR (cm−1, KBr): ν = 3583–3172 (multiple bands, NH2, NH), 1650 (CO), 1610 (CN); 1H NMR (CDCl3): δ (ppm) = 9.33 (s, H, NH, exchangeable), 7.19–7.89 (m, 9H, ArH), 4.55 (br s, 2H, NH2); 13C NMR: δ (ppm) = 161 (C-4, CO), 153 (C-2), ), 149 (C-9), 134(C-7), 128(C-4/), 133 (C-1/), 127(C-6), 126 (C-5,C-8), 121 (C-10); Anal. Calcd for C14H12N4O (252): C, 66.65; H, 4.79; N, 22.21%. Found: C, 67.88; H, 5.40; N, 22.28%.5-Phenyl-1H- [1,2,4] triazino[4,3-a] quinazoline-2,6(3H,5) -dione; 2-Hydroxy-5-phenyl-1H- [1,2,4] triazino [4,3-a] quinazolin-6(5H)-one (3) A mixture of 2 (2.03 g, 5 mmol) and ethyl chloro acetate (0.61 g, 5 mmol) in absolute ethanol (25 mL) was heated under reflux for 10 h. The solid that separated after cooling and recrystallized from dioxan gave (3).Yield, 73%; m.p. 228–230°C; IR (cm−1, KBr): ν = 3449 (OH), 3171 (NH), 1686(CO), 1631(CN); 1H-NMR(DMSO-d): δ (ppm) =13.22 (s,1H,OH, D2O exchangeable), 9.557 (s,1H, NH, D2O exchangeable), 7.52–8.33 (m,9H,Ar-H), 5.49 (s,2H,CH2); 13C NMR: δ (ppm) =162(C-OH), 161 (CO), 153 (C-imine), 141(C-11), 133(C-9), 128(C-7), 117(C-8), 115 (C-12), 107(C-10), 52(C-triazine ring); EI/MS (Scheme 2) m/z: M+ = 292 (0.87), 276 (9.92), 275 (53.62), 263 (1.07), 262 (1.87), 246 (1.13), 235 (3.87), 234 (1.36), 207 (0.92), 194(0.88), 177 (0.93), 166 (0.75), 162 (0.44), 157 (0.83), 145(1.56), 144 (9.32), 131 (1.46), 129 (2.71), 129 (2.71), 124(1.01), 123 (2.77), 119 (3.64), 116 (17.44), 115 (3.26), 110 (4.56), 104 (12.82), 103 (18.15), 102 (13.79), 97 (5.92), 95 (5.10), 92 (2.40), 91 (8.70), 90 (16.29), 89 (32.14), 83 (11.16), 75 (100), 75 (25.08), 65(18.68), 64 (26.11), 56 (27.29), 54 (26.38), 51 (41.41), 42(28.43); Anal. Calcd. for C16H12N4O2 (292): C, 65.75; H, 4.11; N, 19.18%. Found: C, 65.11; H, 4.86; N, 19.77%.2-(2-(4-Hydroxy-3-methoxybenzylidene)-hydrazinyl)-3- phenylquinazolin-4(3H)-one (4) A mixture of 3 (1 g, 10 mmol) and vanillin (10 mmol) in dioxane (15 mL) was heated under reflux for 4 h in the presence of a catalytic amount of acetic acid. The excess dioxane was distilled off, and the reaction solution was left to cool to obtain the solid product which crystallized from a suitable solvent to give (4). Yield, 73%; m.p. 178–180°C; IR (cm−1, KBr) ν = 3516 (OH), 3456 (NH), 2946 (CH, OCH3), 1686 (CO), 1631 (CN), 1H NMR (CDCl3): δ (ppm) = 8.48(s,1H,NH,exchangeable), 7.52–8.03(m,9H,ArH), 6.91(s,1H,N=CH), 5.35 (s,1H, aromatic OH), 3.83 (s,3H,OCH3); 13C NMR: δ (ppm)= 169 (C-4, CO),153 (C-2), 151 (C-OH), 149 (C-9), 147 (C=N), 133 (C-7), 128 (C-5), 127 (C-6), 126 (C-8), 120 (C-10), 56 (O-CH3), Anal. calcd for: C22H18N4O3 (386): C, 68.39; H, 4.66; N, 14.51%. Found: C, 67.11; H, 4.86; N, 15.77%.4-(3-Acetyl-5-oxo-4-phenyl-3,3a,4,5-tetrahydro-[1,2,4-triazolo[4,3-a]-quinazolin-1-yl)-2-methoxy phenyl acetate (5) A mixture of (4) (5 mmol), acetic anhydride (10 mL), and anhydrous sodium acetate (0.41 g, 5 mmol) was heated under reflux for 3 h. The solvent was removed under reduced pressure and the residue was washed with water, filtered, dried, and crystallized from proper solvent to give (5) in good yield (65%). m.p. 151–155°C; IR(cm−1, KBr) ν = 2943 (CH, OCH3), 1700 (CO), 1680 (CO), 1631 (CN); 1H NMR (CDCl3): δ (ppm) = 6.98–8.11 (m, 12H, ArH), 3.83 (s,3H,OCH3); 2.31 (s,3H,OCOCH3), 2.04 (s, 3H, NCOCH3); 13C NMR: δ (ppm) = 169 (OCOCH3), 168 (NCOCH3), 161.3 (CO, quinazoline), 148 (C=N), 137 (C-7), 132 (C-5), 131.4 (C-6), 131 (C-10), 126.3 (C-8), 124 55.8 (O-CH3), 22.8 (NCOCH3), 20.3 (OCOCH3); Anal. Calcd for C26H22N4O5 (470): C, 66.38; H, 4.68; N, 11.91%. Found: C, 66.17; H, 5.59; N, 11.02%.2-(4-Hydroxy-3-methoxyphenyl)-3-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl) amino) thiazolidin-4-one (6).A mixture of (4) (2.6 g, 5 mmol) and 2-mercaptoacetic acid (0.46 g, 5 mmol) was stirred in dry benzene (25 mL) for 15 min, then refluxed for 3 h. The yellow solution was distilled, and the residue was recrystallized from benzene to give (6). Yield, 71% (benzene), m.p. 100–105°C; IR(cm−1, KBr) ν = 3000–2915 (OH), (NH), 2526 (OCH3), 1679 (CO), 1630 (CN); 1H NMR(CDCl3): δ (ppm) = 9.98 (s, H, NH, exchangeable), 7.01–8.12 (m, 12H, ArH), 6.10 (s, H, OH, exchangeable), 4.80 (s, 2H, CH2), 3.72 (s, 1H, methine proton), 2.31 (s, 3H, CH3), 13C NMR: δ=169(CO thiazolidin-4-one ring), 168 (NCOCH3),161 (CO, quinazoline ring), 148 (C=N), 137 (C-7), 132(C-5), 131 (C-6), 131 (C-10), 126.3 (C-8), 124 55.8(O-CH3), 22.8 (NCOCH3), 20.3 (OCOCH3) Anal. Calcd for C24H20N4O4S (460): C, 62.60; H, 4.38; N, 12.17%. Found: C, 62.02; H, 5.32; N, 12.34%.
3.3. Determination of Antioxidant Activity (DPPH Scavenging Activity)
- The free-radical scavenging activities were assayed using 2-diphenyl-1-picrylhydrazyl (DPPH) Table 2, in which the antioxidant compound scavenged the free radicals that were released from DPPH [44]. The different samples were prepared in different concentrations (0.25, 0.5, 0.75, 1 mg/mL). A quantity of 100 µL of DPPH (4 mg/100 mL methanol) solution was mixed with 100 µL of the samples at different concentrations, each separately. Absorbance at 517 nm was determined after 30 min of dark incubation. Each experiment was performed in triplicate and the average was taken. Vanillin was used as a positive control and the percentage of free-radical scavenging activity was calculated using the following formula: % DPPH of radical scavenging activity = [(A control − A sample)/A control] × 100.
3.4. Determination of Anti-Inflammatory Activity (Nitric Oxide Scavenging Activity)
- Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitric ions that can be estimated by using a Griess reagent [50]. The reagents used were sodium nitroprusside (10 mM), phosphate buffer saline and Griess reagent (1 g of sulphanilic acid + 0.1 g naphthylethylene diamine dihydrochloride). A quantity of 20 μL sodium nitroprusside, 5 μL phosphate buffer and 5 μL of the different concentrations of the samples were incubated at 25°C for 2.30 h. After incubation, 20 μL of Griess reagent was added to the previous mixture and allowed to stand for 30 min. The absorbance of the color developed was observed at 550 nm on the spectrophotometer. Each experiment was done in triplicate and the average was taken. Vitamin C was used as a positive control and the percentage of free-radical scavenging activity was calculated from the formula: % of nitric oxide scavenging activity = [(A control − A sample) /A control] × 100.
3.5. Computational Details
- All calculations for the studied compounds were carried out using the Gaussian 09 software package [65] with the processor: Intel (R) Core (TM) i7 personal computer. The calculations were performed by DFT using the Becke’s three-parameter sex-change functional with a hybrid functional by means of the Lee, Yang and Parr LYP correlation functional [51] B3LYP method. No constraints were applied to the calculations. The energy optimization of the ground state of the compounds under investigation was performed using the split-valence basis sets 6-311G (d, p) and 6-31++G (d, p) with two polarized basis functions (d, p), where the p-type orbital was added to all hydrogen atoms, as well as the diffuse function. The Gauss-View [65] and Chem Craft programs [66] were used to obtain the computational results, to visualize the optimization structures and to draw the frontier molecular orbitals (FMOs) and molecular electrostatic potential (MEP) maps [67].
4. Conclusions
- Different heterocyclic compounds were synthesized with quinazolin-4(3H)-ones as the core. The synthesized compounds were isolated in good yields and their structures were confirmed using different spectral analyses, such as IR, 1H-NMR, 13C-NMR and mass spectra. The antioxidant properties of these molecules were evaluated experimentally by both DPPH radical and nitric oxide scavenging activity methods, and theoretically using DFT/B3LYP at 6-311G (d, p) and 6-31++G (d, p) basis sets. Chemical modifications, together with density functional theory study on the target compounds help us to confirm some valuable activities against free radicals. Based on the results of the present study, the antioxidant activities of the studied compounds appear to be related to the presence of the quinazolin-4(3H)-one ring with different fused heterocyclic rings in compounds (2–5) and the thiazole ring in compound (6).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML