-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2019; 9(1): 9-13
doi:10.5923/j.ajoc.20190901.02

Synthesis, Characterization, Antioxidant and Antimicrobial Activity of Copper(II) Complex with Schiff Base Derived from 2,2-dihydroxyindane-1,3-dione and Tryptophan
Emir Horozić1, Jasmin Suljagić1, Mersiha Suljkanović2
1Faculty of Technology, University of Tuzla, Tuzla, Bosnia and Herzegovina
2Faculty of Natural Sciences and Mathematics, Tuzla, Bosnia and Herzegovina
Correspondence to: Emir Horozić, Faculty of Technology, University of Tuzla, Tuzla, Bosnia and Herzegovina.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Schiff bases and complexes are today the subject of many studies because of the established biological, inhibitor and catalytic properties. The aim of this paper is to investigate the interaction of Schiff base obtained by reaction of 2,2-dihydroxyindane-1,3-dione (ninhydrin) and the essential amino acid tryptophan with copper(II) ion. Spectral characterization and examination of the potential antimicrobial and antioxidant activity of the synthesized complex were performed. The imine Cu(II) complex is characterized by FTIR and UV/Vis spectroscopy. Stoichiometric M:L ratio was determined by Joe and Yones method. Antioxidant activity was tested by DPPH and FRAP method. Antibacterial and antifungal activity was determined by diffusion technique on reference strains from the ATCC collection. The results showed that the synthesized Schiff base coordinates the Cu(II) ion as a tridentate ligand, in a molar ratio of 1:2 (M:L). The synthesized complex showed significant antioxidant activity. The antimicrobial effect of the Cu(II) complex in the case of S. aureus, E. faecalis, L. monocytogenes, B. subtilis and C. albicans was obtained, with inhibition zones of 11-20 mm.
Keywords: Copper, Schiff base, FTIR, UV/Vis, Antimicrobial activity
Cite this paper: Emir Horozić, Jasmin Suljagić, Mersiha Suljkanović, Synthesis, Characterization, Antioxidant and Antimicrobial Activity of Copper(II) Complex with Schiff Base Derived from 2,2-dihydroxyindane-1,3-dione and Tryptophan, American Journal of Organic Chemistry, Vol. 9 No. 1, 2019, pp. 9-13. doi: 10.5923/j.ajoc.20190901.02.
Article Outline
1. Introduction
- Schiff bases are synthetically accessible and structurally diverse compounds, typically obtained by condensation between an aldehyde or a ketone with primary amines [1]. Schiff base ligands are essential in the field of coordination chemistry, especially in the development of complexes of Schiff bases because these compounds are potentially capable of forming stable complexes with metal ions [2].
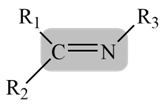 | Figure 1. General structure of Schiff base |
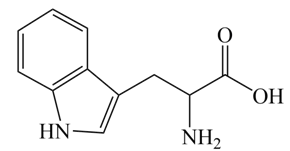 | Figure 2. Structure of Tryptophan |
2. Experimental
- All chemicals were of reagent grade, where possible, purchased from Aldrich and used without further purification.
2.1. Synthesis of the Complex
- Synthesis of the complex was carried out according to the previously published procedure [10]. 0.01 mole of 2,2-dihydroxyindane-1,3-dione (L1) was dissolved in 30 mL of ethanol and 0.01 mole of CuCl2 x 2H2O salt was added to the resulting solution, which was shaken and heated until all of it dissolved. This mixture was refluxed for about half an hour, followed by addition of 0.01 mole tryptophan (L2) to the hot mixture and the mixture was refluxed for a two hours. The resulting brown colored precipitate was then filtered off. The product was then dried and stored in a desiccator. Yield: 65%. Melting point of product is 220.5°C.
2.2. Spectral Characterization
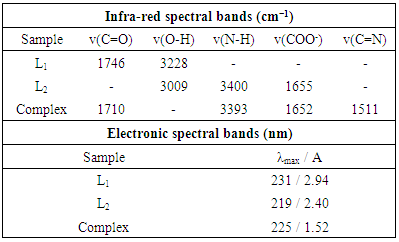
- In order to determine structure of the complex, samples were recorded on Nicolet iS10 FT-IR spectrophotometer - Thermo Fisher Scientific. The ATR technique was used for sample analysis. Samples were recorded in the range of 4000-650 cm-1.Aqueous Cu(II) solutions at concentration of 0.6 x 10-3 mol L-1 were used to recording UV spectra. The metal salt, 2,2-dihydroxyindane-1,3-dione and tryptophan were added in the same volume ratio, mixed for 2 hours at 300 rpm, after which UV spectra were recorded. Absorption spectra were recorded on a double-bean UV/Vis spectrophotometer Perkin Elmer λ25, in the wavelength range of 200-400 nm [11].
2.3. Determination of Stoichiometric Ratio
- Stoichiometric ratio M:L was tested using Joe and Yones method [12]. A series of solutions with a constant concentration of Cu(II) ions of 1.35 x 10-4 mol L-1 were prepared, while ligand concentration was changed. Based on the obtained results, the stoichiometric composition of the complex and the calculated stability value of the KML were determined.
2.4. Morphological Characterization
- Prior to morphological characterization, solid complexes were treated with DMSO. The color, size and shape of Cu(II) crystal complex were determined by microscopic analysis, performed with the binocular microscope, the Leica DM 2500P mark.
2.5. Determination of Antioxidant Capacity
- 2,2-diphenyl-1-picryl-hydrazyl (DPPH) method was performed according to earlier described method [13]. The radical scavenging effect (%) or percent inhibition of DPPH radical was calculated according to the equation: [(Acontrol-Asample)/Acontrol] x 100where Asample is the absorbance of the solution containing the sample at 517 nm and Acontrol is the absorbance of the DPPH solution. The results are expressed as the IC50 value (mg mL-1) or the concentration of extract that caused 50% neutralization of DPPH radicals.The determination of ferric reducing antioxidant power or ferric reducing ability (FRAP assay) was performed as described earlier [14]. To prepare the calibration curve, solutions of FeSO4 x 7H2O were prepared in the concentration range of 200-1000 μmol L-1 (y = 0.001x + 0.0615; R² = 0.9907). In each tube, 0.1 mL of Cu(II) complex and 3 mL of FRAP reagent were added. The samples were incubated in an aqueous bath for 30 minutes at 37°C, and the absorbance was measured at 593 nm.
2.6. Antimicrobial Activity in vitro
- Antimicrobial activity was analyzed following the published procedures [15,16]. Antibacterial activity were investigated by diffusion method on reference bacterial strains E. coli, E. faecalis, S. aureus, B. subtilis, L. monocytogenes and P. aeruginosa. Antifungal activity of the complex was tested on Candida albicans. From the microorganisms strains of overnight cultures, suspensions of 0.5 McFarland turbidity were prepared (density 107-108 CFU mL-1). The strains were then placed on the surface of the nutrient substrate-Mueller-Hinton agar, dispersed in sterile Petri dishes. Substrate thickness was 4 mm. In the agar sterile drill-shaped holes were made ("wells"), into which 80 μL of Cu(II) complex solutions in concentration of 5 mg mL-1 were added. After the plates were left at room temperature for 15 minutes, the substance was diffused into agar, incubated at 37°C/24 h. After the incubation period, the size of the inhibitory zone was measured, and the sensitivity of the microorganisms was expressed in the manner described above [17].
3. Results and Discussion
3.1. Reaction Scheme and Structure of Complex
- The reaction scheme and the proposed structure of the Cu(II) complex is shown at Figure 3. It is assumed that the synthesized Schiff base coordinates the copper ion as the tridentate ONO ligand. The formation of the bond with metal involves the oxygen atom of the carbonyl group of the indane part of the molecule and the other from the deprotonated carboxyl group of tryptophan. A third bond is formed between the metal ion and the nitrogen atom of the imine group.
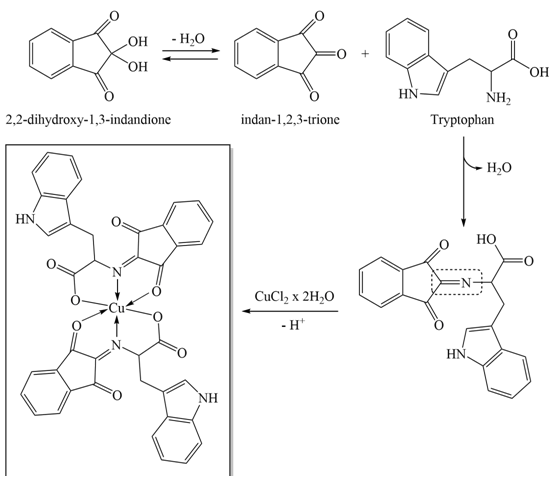 | Figure 3. Reaction scheme and proposed structure of the complex |
3.2. Spectral Characteristics
- Spectral characteristics of reactants and Cu(II) complex were described at Table 1.
|
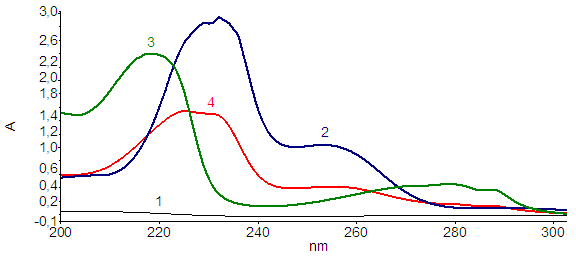 | Figure 4. UV spectra: (1) Cu(II) ion; (2) Ninhydrin; (3) Triptophan; (4) Cu(II) complex |
3.3. Stoichiometric Ratio
- The solutions of metal salt and Schiff base were prepared by dissolving them in an ethanol-water mixture in volume ratio 3/1. The spectra of the complex used to determine the stoichiometric ratio are shown in Figure 5. Absorbance vs. molar ratio (M/L) graph is shown in Figure 6. From the obtained data, the calculated stability constant of the complex KML2 is 2.83 x 107.
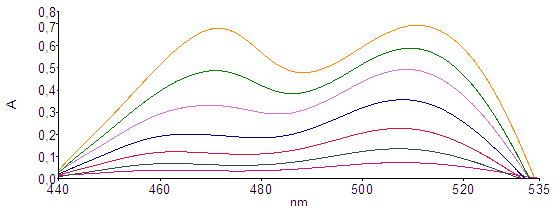 | Figure 5. Absorption spectra of the Cu(II) complex |
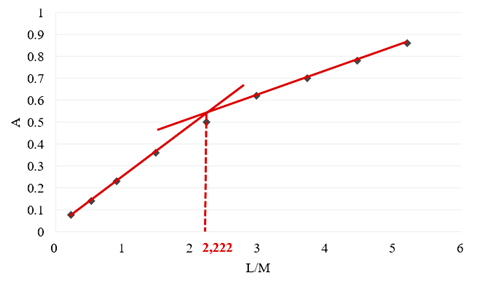 | Figure 6. Absorbance vs. molar ratio (M/L) diagram |
3.4. Morphological Characteristics
- The crystals of the synthesized Cu(II) complex (Figure 7) are round-shaped forms, with a characteristic radial-air aggregates (from a common central part, aggregates are circularly matched) and diameter of up to 0.15 mm. Interferential colors are live of the first order.
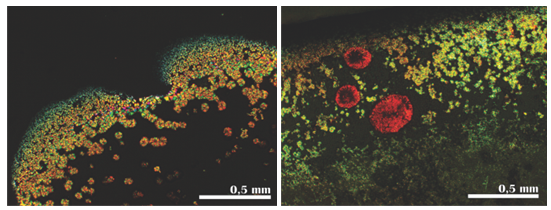 | Figure 7. Morphology of Cu(II) complex crystals |
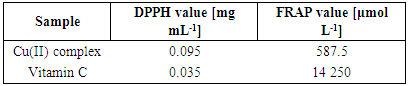
3.5. Antioxidant Activity in vitro
- The calibration curves obtained by the DPPH method for complex and vitamin C are shown in Figures 8 and 9. Based on the IC50 value, it has been established that the Cu(II) complex has a high antioxidant capacity. Vitamin C has a slightly better antioxidant effect (IC50 = 0.035 mg mL-1).
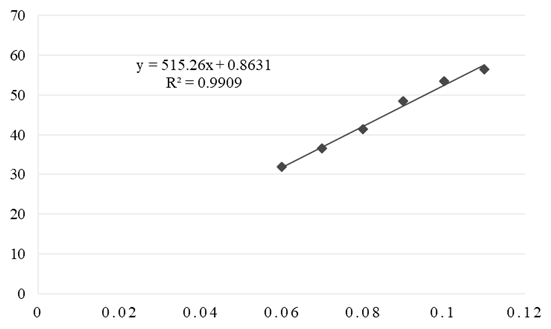 | Figure 8. Calibration curve of Cu(II) complex obtained by DPPH method |
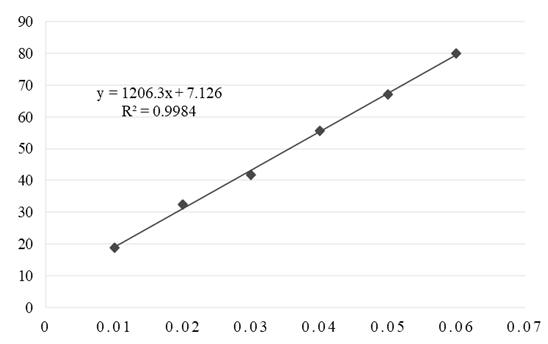 | Figure 9. Calibration curve of vitamin C obtained by DPPH method |
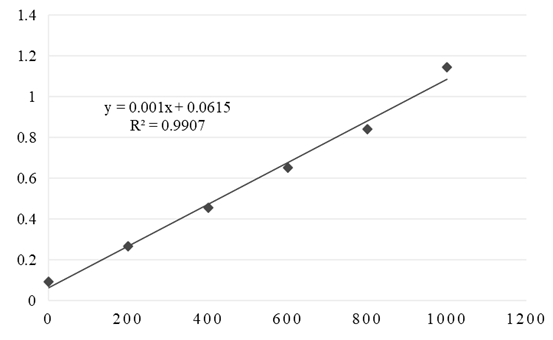 | Figure 10. Calibration curve for FeSO4 x 7H2O |
|
3.6. Antimicrobial Activity in vitro
- The results of antimicrobial activity are shown in Table 3. Ciprofloxacin and Nystatin were used as controls. The Cu(II) complex has no antibacterial action against gram-negative bacteria (E. coli and P. aeruginosa). Poor activity was recorded in E. faecalis and B. subtilis. Significant action was observed in other strains, with inhibition zones of 16 to 20 mm. However, Ciprofloxacin showed significant antibacterial activity in relation to the complex, at a lower concentration (1 mg/mL). The complex showed similar activity as to Nystatin in the case of C. albicans, with an inhibition zone of 20 mm.
|
4. Conclusions
- Schiff base derived from 2,2-dihydroxyindane-1,3-dione and Tryptophan coordinates Cu(II) ion as a tridentate ONO donor ligand, in a stoichiometric ratio of 1:2 (M:L). Crystals of complexes are round-shaped forms. The synthesized complex has a significant antioxidant activity. Significant antimicrobial activity was found in the case of C. albicans, L. monocytogenes and S. aureus, while lower antimicrobial activities were found for other tested microorganisms.
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge support from the grant of the University of Tuzla, Bosnia and Herzegovina in 2018.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML