-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2019; 9(1): 1-8
doi:10.5923/j.ajoc.20190901.01

Synthesis, Reactions and Biological Importance of α, β-Unsaturated Carbodithioate Esters: A Review
Md. Ashraful Alam1, 2, Kazuaki Shimada2, Yusuke Taneichi2, Md. Wahab Khan1, Md. Abdur Rashid1, Md. Chanmiya Sheikh3
1Department of Chemistry, Bangladesh University of Engineering and Technology (BUET), Dhaka, Bangladesh
2Department of Chemistry and Biological Sciences, Iwate University, Morioka, Japan
3Department of Applied Chemistry, University of Toyama, Toyama, Japan
Correspondence to: Md. Ashraful Alam, Department of Chemistry, Bangladesh University of Engineering and Technology (BUET), Dhaka, Bangladesh.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Carbodithioate esters are important functional organosulfur compounds widely used in different fields such as pharmaceuticals, agrochemicals and material sciences. α, β-unsaturated dithioesters, carbodithioates are the type of organosulfur chemical compounds that attract the special interest of organic chemists. α, β-unsaturated dithioester type compounds have various applications in medicinal chemistry and provided numerous potent α, β-unsaturated dithioester derivatives for different therapeutic targets. α, β-unsaturated dithioesters are widely used as solvents, polymers, and biopharmaceutical agents. For the treatment of tuberculosis, leprosy and dermatitis herpetiformis diseases, several drug molecules containing α, β-unsaturated dithioester groups are used. Based on these applications, researchers have engaged in the preparation and study of many types of α, β-unsaturated dithioester derivatives for their medicinal activities e.g. biological, antimalarial, antimicrobial, anti-inflammatory, anticancer, anti-HIV, and anti-inflammatory properties. The present article provides a targeted review of recent synthetic strategies, pertinent reactions and applications of α, β-unsaturated dithioester type compounds to facilitate future research efforts so that the medicinal and industrial applications of this important class of compounds continue to be beneficial to society.
Keywords: α, β-unsaturated carbodithioates, Synthesis, Anticancer activity, Biological importance, Medicinal chemistry
Cite this paper: Md. Ashraful Alam, Kazuaki Shimada, Yusuke Taneichi, Md. Wahab Khan, Md. Abdur Rashid, Md. Chanmiya Sheikh, Synthesis, Reactions and Biological Importance of α, β-Unsaturated Carbodithioate Esters: A Review, American Journal of Organic Chemistry, Vol. 9 No. 1, 2019, pp. 1-8. doi: 10.5923/j.ajoc.20190901.01.
Article Outline
1. Introduction
- Carbon-sulfur bond formation is a fundamental approach to introduce sulfur into organic compounds. Carbon-sulfur bond formation has received considerable attention due to the prominence of the C-S bond in various molecules that are of biological, pharmaceutical and material interest [1].α, β-unsaturated thioesters have attracted much attention as active esters for the syntheses of different compounds. Synthetic methods for α, β-unsaturated dithioesters have received considerable interest in view of their increased reactivity, compared to their carboxylic analogues, as potential dienes or dienophiles in hetero Diels–Alder cycloadditions [2]. Moreover, the cycloaddition products, thiochromenes, are potential precursors of a wide range of thioheterocycles with interesting biological properties. There are very few general methods available for the synthesis of α, β-unsaturated dithioesters and those known are mostly specific to certain substrate classes. The methods available in the literature include (i) alkylation of thiolate anions obtained by the addition of vinyl cuprates to carbon disulfide [3], (ii) isomerization of α, β-unsaturated dithioesters [4], (iii) base catalyzed elimination of β-hydroxy dithioesters [5], and (iv) Wittig–Horner, Peterson or Mukaiyama type condensation reactions of aldehydes and ketones [6]. Hartke et al. have also shown that α, β-unsaturated amides can be transformed into the corresponding dithioesters by a sequence of reactions involving thionation, alkylation and sulfhydrolysis [7]. Although dithioesters have been known for many years [8], it is only recently that α, β-unsaturated dithioesters have attracted attention. We were interested in that α, β-unsaturated dithioesters as potential heterodienes or in cycloaddition reactions [9].
2. Synthesis and Reactions
- In 1978, the preparation of the β-hydroxydithioester 1 was described [10] involving the treatment of ethyl dithioacetate with lithium di-isopropylamide (LDA) in tetrahydrofuran (THF) followed by isobutyraldehyde at - 78°C. It is recently that α, β-unsaturated dithioesters have attracted attention. Preparative approaches to these compounds 2 which have been investigated include: (a) reaction of a vinyl cuprate with carbon disulphide followed by methyl iodide [11]; this is successful for compounds (2a-c), but attempts to make the dithioester (2d) in this way gave its dimer; (b) sulphydrolysis at -75°C of the immonium salt derived by S-methylation of the thioamide gave the phenyl derivative (2e) [12] which dimerized above -30°C; (c) base-catalysed isomerisation of β,γ-unsaturated dithioesters [13], in turn prepared from N-phenyliminothioesters, gave (2a) and, at -40°C, (2f) which dimerized at room temperature; and flash pyrolysis of the bridged anthracene and trapping of the product in a matrix at -196°C gave the parent dithioacrylate (2g). K. R. Lawson et al. were interested in α, β-unsaturated dithioesters as potential heterodienes or heterodienophiles in cycloaddition reactions [14], and they describe their preparation from β-hydroxydithioesters and some cycloadditions in which they are involved. Subsequent to the completion of their work it was reported that addition of Grignard reagents to β-ketodithioesters gave 3,3-disubstituted β-hydroxydithioesters, and the latter were dehydrated to 3,3-disubstituted α, β-unsaturated dithioesters on treatment with toluene-p-sulphonic acid in benzene at reflux [15].
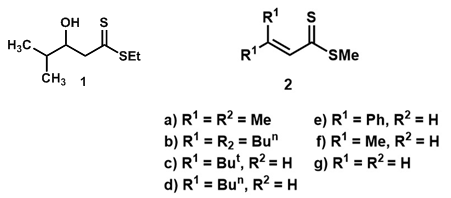 | Figure 1. β-hydroxydithioester and α, β-unsaturated dithioesters |
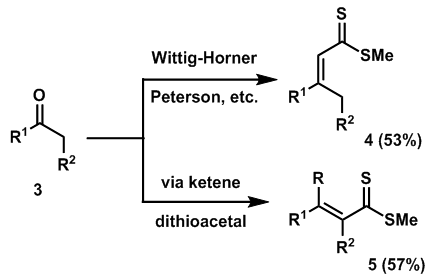 | Scheme 1. Synthesis of α, β-unsaturated dithioesters through Wittig-Horner method and via ketene dithiacetal process |
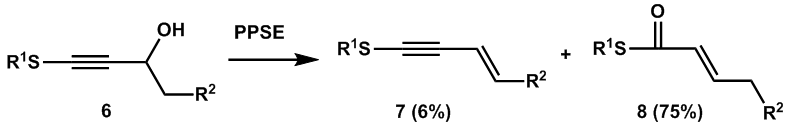 | Scheme 2. Meyer-Schuster rearrangement of γ-sulfur-substituted propargyl alcohols to synthesis of α, β-unsaturated thioesters in 1995 |
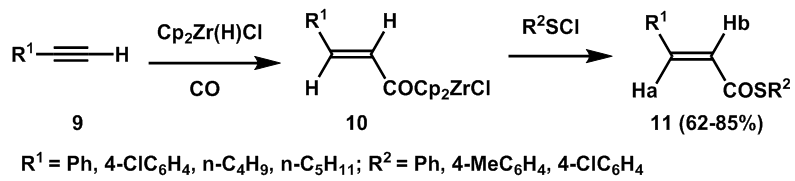 | Scheme 3. A stereoselective synthetic route to (E)-α, β-unsaturated thioesters |
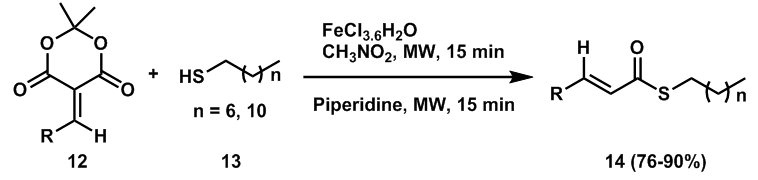 | Scheme 4. One-pot direct synthesis of α, β-unsaturated thioesters |
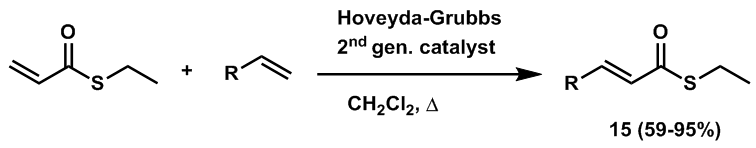 | Scheme 5. Cross-metathesis reaction of S-ethyl thioacrylate with a variety of olefins to give substituted α, β-unsaturated thioesters |
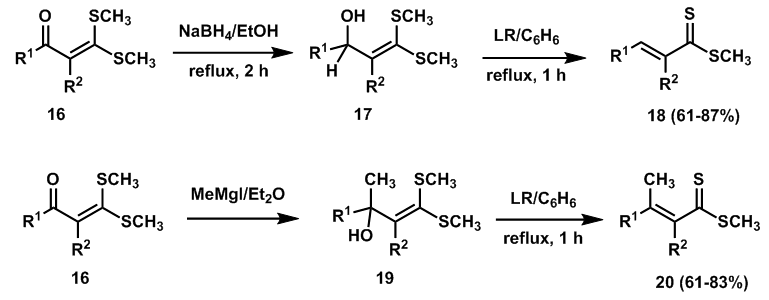 | Scheme 6. Synthesis of α, β-unsaturated dithioesters from the reactions of α-hydroxyketene dithioacetals with Lawesson’s reagent |
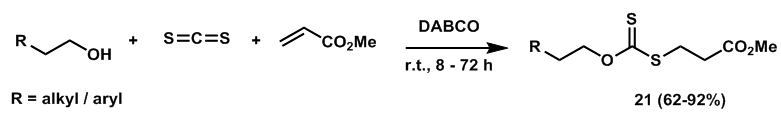 | Scheme 7. Synthesis of functionalized O, S-dialkyldithiocarbonates |
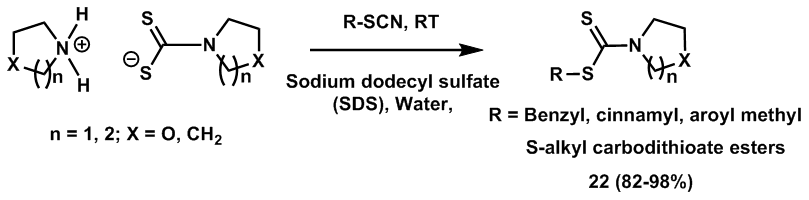 | Scheme 8. Synthesis of carbodithioate esters from organyl thiocyanates |
3. Biological Importance of α, β-Unsaturated Carbodithioate Esters
- There is a malignant disease named acute myelogenous leukemia (AML) which is characterized by an aberrant accumulation of immature myeloid haematopoietic cells [55]. This AML is the most common form of acute leukemia in adults and constitutes approximately 80% of cases [56]. Though the treatments of AML significantly improved the rate of remission, still more than 50% relapse with to a resistant form of the disease. So still there is challenge for this AML chemotherapy [57]. Another group of leukemic cells, (Leukemia stem cells (LSCs)) have shown self-renewal ability as well as the capability to produce heterogeneous leukemia cell populations [58, 59]. It has been considered to play significant role in the initiation and relapse of acute leukemia [60]. LSCs are also considered to be an effective strategy for the treatment and possible cure of AML [61-640]. Also, LSCs are refractory to clinical used chemotherapy drugs, such as nucleoside analogues like cytosine arabinoside and anthracyclines like idarubicin and daunorubicin [65, 66]. So, the effective agents that can selectively eradicate LSCs are urgently needed for the development of new therapies for treatment of leukemia.Using this information, Dinga, Y. et al. designed, synthesized, and evaluated a series of dithiocarbamate esters of parthenolide (PTL) for their anti-AML activities [67]. Among the most promising compound 23 showed greatly improved potency against AML progenitor cell line KG1a with IC50 value of 0.7 μM, and the efficacy found 8.7-folds comparing to that of PTL (IC50= 6.1 μM). The compound 23 induced the apoptosis of total primary human AML cells and leukaemia stem cell (LSCs) of primary AML cells while sparing the normal cells. The compound 23 suppressed the colony formation of primary human leukaemia cells. The preliminary molecular mechanism study revealed that the compound 23-mediated apoptosis is associated with mitogen-activated protein kinase signal pathway. After some of the research results, Dinga, Y. et al. proposed that the compound 24 also might be a promising drug candidate for ultimate discovery of anti-LSCs drug (Figure 2).
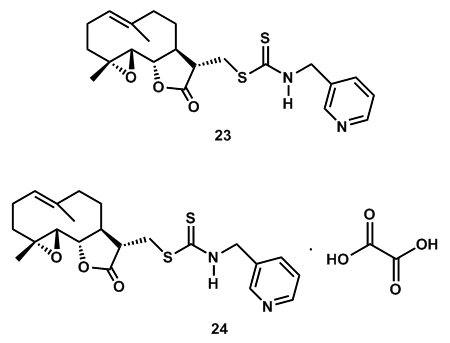 | Figure 2. Dithiocarbamate esters 23, showed potency against AML progenitor cell line KG1a and 24, a promising drug candidate for the discovery of anti-LSCs drug |
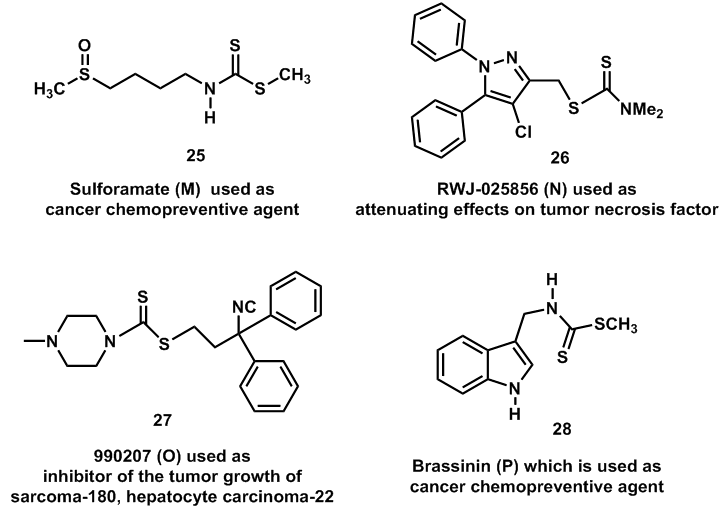 | Figure 3. Carbodithioate esters which have the strong potential therapeutic values |
4. Conclusions
- This review has highlighted the synthesis, reactions and biological importance of α, β-unsaturated carbodithioesters type of compounds and their derivatives. α, β-unsaturated carbodithioesters and their derivatives have myriad applications in biological, pharmaceutical, medicinal and in many other fields. This class of sulfur-containing compounds will continue to be investigated in the future and new applications for these dithioesters will continue to be developed.
ACKNOWLEDGEMENTS
- The authors are grateful to the Department of Chemistry and Biological Sciences, Faculty of Science and Engineering, Iwate University, Japan.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML