-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2018; 8(1): 8-12
doi:10.5923/j.ajoc.20180801.02

Monomodal vs Multimodal Microwave Irradiation Applied in the Synthesis of Fluorochalcones
José Eladio Antonio-Arias1, Verónica del C. Díaz-Oliva1, Nancy Romero-Ceronio1, Abraham Gómez-Rivera1, Hidemi Aguilar-Mariscal2, Luis F. Roa de la Fuente1, Carlos E. Lobato-García1
1División Académica de Ciencias Básicas. Universidad Juárez Autónoma de Tabasco, Tabasco, México
2Laboratorio de Farmacología, Unidad de Protección, Cuidado y Experimentación de Animales, Universidad Juárez Autónoma de Tabasco, Tabasco, México
Correspondence to: Nancy Romero-Ceronio, División Académica de Ciencias Básicas. Universidad Juárez Autónoma de Tabasco, Tabasco, México.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The synthesis of o-, m- and p-fluorine-substituted chalcones at the ring “B” was accomplished by a Claisen-Schmidt condensation between the benzaldehyde and acetophenone. The reaction was performed in solvent-free conditions with microwave activation and good yields (> 75%) were obtained. It is noteworthy that the application of conventional reaction conditions produced very low yields and in some cases, the reaction did not proceed at all. The methodology implemented considerably reduces reaction times.
Keywords: Fluorine-substituted Chalcones, Claisen-Schmidt Condensation, Solvent-free reaction
Cite this paper: José Eladio Antonio-Arias, Verónica del C. Díaz-Oliva, Nancy Romero-Ceronio, Abraham Gómez-Rivera, Hidemi Aguilar-Mariscal, Luis F. Roa de la Fuente, Carlos E. Lobato-García, Monomodal vs Multimodal Microwave Irradiation Applied in the Synthesis of Fluorochalcones, American Journal of Organic Chemistry, Vol. 8 No. 1, 2018, pp. 8-12. doi: 10.5923/j.ajoc.20180801.02.
Article Outline
1. Introduction
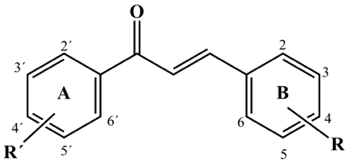
- Chalcones constitute an important group of biomolecules, some of which exhibit a wide range of biological activities [1] for example: antifungal [2], antibacterial [3], anti-inflammatory [4, 5], antitumor [6], and antioxidant properties [7].Structurally, the chalcone moiety is formed by two aromatic rings bonded by a three carbon skeleton which is present as a carbonyl α, β-insaturated system (Figure 1) [8].
 | Figure 1. General structure of the chalcone moiety |
2. Experimental
- The synthesis of the three fluorochalcones (2a, 2b and 2c) was conducted by the Claisen-Schmidt condensation of o-fluorobenxaldehyde (1a), m-fluorobenzaldehyde (1b) and p-fluorobenzaldehyde (1c) with acetophenone. All the substances were analytical-grade reagents and were employed without further purification. The reactions were performed through conventional (use of solvents, stirring and/or reflux conditions) and green chemistry (free-solvent conditions, microwave irradiation) procedures (Figure 2).
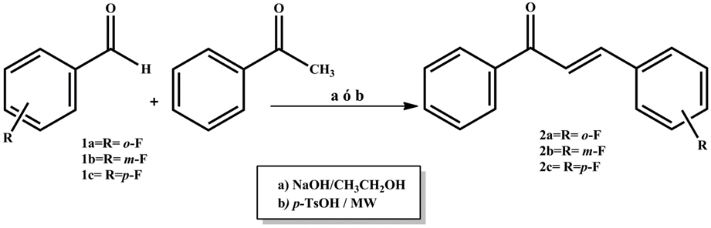 | Figure 2. Reaction scheme for the synthesis of compounds 2a-2c. Reaction conditions: a) NaOH/CH3CH2OH, continuous stirring and/or reflux; b) p-TsOH, free solvent, microwave activation |
2.1. General Procedure for Conventional Synthesis of Chalcones 2a-c
- The conventional procedure was performed by dissolving sodium hydroxide (0.6 eq. aq. 0.1 mMol) in ethanol (1mL), this mixture was put into an ice-bath at 0°C; once this temperature was reached, acetophenone (1 equivalent) and the corresponding fluorobenzaldehyde (1 equivalent) were slowly added. The reaction mixture was removed from the ice-bath and maintained with magnetic stirring until no further changes were observed. The reaction was monitored by TLC (silica gel 60 F254, hexane/ethyl acetate 95:5). Where no substantial changes were observed, the reaction mixture was heated under reflux conditions. The reaction crude was cooled at 0°C during 24 h; and the solid products were filtered and washed with cold ethanol. The crystallization was made from an ethanol/dichloromethane mixture. The recrystallized products were dried, weighed and characterized.
2.2. General Procedure for the Solvent-Free Synthesis of Chalcones 2a-c
- The eco-friendly synthetic procedures were performed in solvent-free conditions and acid catalysis. A mixture of p-toluensulfonic acid (PTSA) (0.5 eq), acetophenone (1 eq) and the corresponding fluorobenzaldehyde (1 eq) were placed in a proper reactor. The system was microwaved irradiated employing a monomodal microwave system (MW) (VICHI, model: MW-600 MIC-1). For comparative purposes, a domestic microwave oven (MO) was also employed (MABE, model: HMM74MB). The reaction was monitored by TLC (silica gel 60 F254, hexane/ethyl acetate 95:5). The reaction crude was purified by CC (silica 60 mesh, hexane/ethyl acetate 98:2). The eluents were removed by vacuum distillation. The solid products were recrystallized from a mixture of hexane/ethyl acetate. The purified products were dried, weighed and characterized.
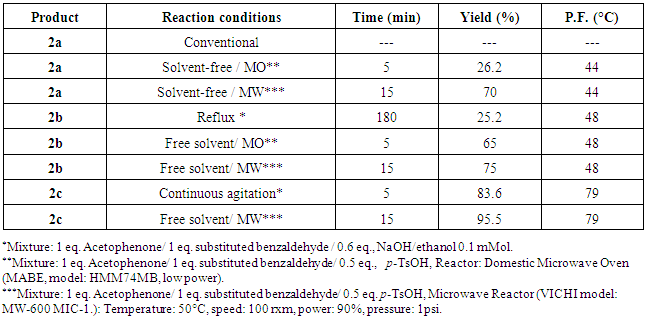
2.3. (E)-3-(2-Fluorophenyl)-1-Phenylprop-2-en-1-one, 2a
- Pure product 2a was obtained as a yellow solid (highest yield = 70%); m.p. 44°C (reported: 38° to 40°C, [25]). Spectroscopic analysis:
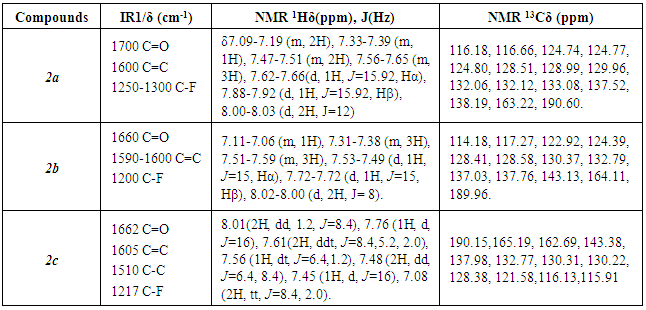
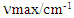 (neat KBr) = 1700 (s), 1600 (s), 1250-1300 (s). 1H NMR (400 MHz, CDCl3): δ7.09-7.19 (m, 2H), 7.33-7.39(m, 1H), 7.47-7.51 (m, 2H), 7.56-7.65 (m, 3H), 7.62-7.66(d, 1H, J=15.92 Hz, Hα), 7.88-7.92 (d, 1H, J=15.92 Hz), 8.00-8.8.03 (d, 2H); 13C NMR (100 MHz, CDCl3): 116.18, 116.66, 124.74, 124.77, 124.80, 128.51, 128.99, 129.96, 132.06, 132.12, 133.08, 137.52, 138.19, 163.22, 190.60.
(neat KBr) = 1700 (s), 1600 (s), 1250-1300 (s). 1H NMR (400 MHz, CDCl3): δ7.09-7.19 (m, 2H), 7.33-7.39(m, 1H), 7.47-7.51 (m, 2H), 7.56-7.65 (m, 3H), 7.62-7.66(d, 1H, J=15.92 Hz, Hα), 7.88-7.92 (d, 1H, J=15.92 Hz), 8.00-8.8.03 (d, 2H); 13C NMR (100 MHz, CDCl3): 116.18, 116.66, 124.74, 124.77, 124.80, 128.51, 128.99, 129.96, 132.06, 132.12, 133.08, 137.52, 138.19, 163.22, 190.60.2.4. (E)-3-(3-Fluorophenyl)-1-Phenylprop-2-en-1-one, 2b
- Pure product 2b was obtained as a yellow solid (highest yield = 75%); m.p. 48°C (reported: 48°C, [26]). Spectroscopic analysis:
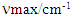 (neat KBr) = 1660 (s), 1590-1600 (s), 1200 (s). 1H NMR (400 MHz, CDCl3): 7.11-7.06 (m, 1H), 7.31-7.38 (m, 3H), 7.51-7.59 (m, 3H), 7.53-7.49 (d, 1H, J=15 Hz, Hα), 7.72-7.72 (d, 1H, J=15 Hz, Hβ), 8.02-8.00 (d, 2H, 15 Hz). 13C NMR (100 MHz, CDCl3): 114.18, 117.27, 122.92, 124.39, 128.41, 128.58, 130.37, 132.79, 137.03, 137.76, 143.13, 164.11, 189.96.
(neat KBr) = 1660 (s), 1590-1600 (s), 1200 (s). 1H NMR (400 MHz, CDCl3): 7.11-7.06 (m, 1H), 7.31-7.38 (m, 3H), 7.51-7.59 (m, 3H), 7.53-7.49 (d, 1H, J=15 Hz, Hα), 7.72-7.72 (d, 1H, J=15 Hz, Hβ), 8.02-8.00 (d, 2H, 15 Hz). 13C NMR (100 MHz, CDCl3): 114.18, 117.27, 122.92, 124.39, 128.41, 128.58, 130.37, 132.79, 137.03, 137.76, 143.13, 164.11, 189.96.2.5. (E)-3-(4-Fluorophenyl)-1-Phenylprop-2-en-1-one, 2c
- Pure product 2c was obtained as a yellow solid (highest yield= 95.5%); m.p. 79°C (reported: 78.85°C, [27] and 83 to 84°C, [28]). Spectroscopic analysis:
 (neat KBr)= 1662 (s), 1605 (s), 1510 (s); 1217 (s). 1H NMR (400 MHz, CDCl3):8.01(2H, dd,1.2, J=8.4 Hz), 7.76 (1H,d, J=16Hz), 7.61(2H, ddt, J=2.0,5.2,8.4 Hz), 7.56 (1H, dt, J=1.2,6.4 Hz), 7.48 (2H, dd, J=6.4, 8.4 Hz), 7.45 (1H, d, J=16 Hz), 7.08 (2H, tt, J=2.0,8.4 Hz). 13C NMR (100 MHz, CDCl3): 190.15, 165.19, 162.69, 143.38, 137.98, 132.77, 130.31, 130.22, 128.38, 121.58, 116.13, 115.91.
(neat KBr)= 1662 (s), 1605 (s), 1510 (s); 1217 (s). 1H NMR (400 MHz, CDCl3):8.01(2H, dd,1.2, J=8.4 Hz), 7.76 (1H,d, J=16Hz), 7.61(2H, ddt, J=2.0,5.2,8.4 Hz), 7.56 (1H, dt, J=1.2,6.4 Hz), 7.48 (2H, dd, J=6.4, 8.4 Hz), 7.45 (1H, d, J=16 Hz), 7.08 (2H, tt, J=2.0,8.4 Hz). 13C NMR (100 MHz, CDCl3): 190.15, 165.19, 162.69, 143.38, 137.98, 132.77, 130.31, 130.22, 128.38, 121.58, 116.13, 115.91.3. Results and Discussion
- Chalcones 2a-2c were prepared by a conventional procedure with basic catalysis in ethanol with mechanical stirring at room temperature and/or reflux conditions. A solvent-free procedure was also employed for the preparation of these compounds with acid catalysis using PTSA and MW or MO as energy source. Table 1, presents a resume of: reaction conditions, reaction yields, and physical appearance of products 2a-2c. All of them were obtained as yellow solids, and their melting point varies from 44 to 79°C. The solvent-free synthesis mediated either by monomodal (MW) or domestic (MO) microwave irradiation presented higher yields (>65%) when compared with both the mechanical agitation and the reflux methods. It is noteworthy that for compound 2a neither the conventional nor the domestic microwave irradiation procedures were suitable for its synthesis, which was only successful by monomodal microwave irradiation.
|
|
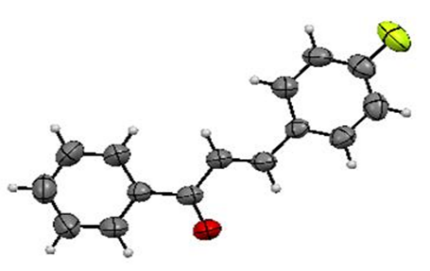 | Figure 3. Molecular structure of 2c, with 30% probability displacement ellipsoids for non-H atoms |
4. Conclusions
- The solvent-free/microwave activation strategy proved to be a satisfactory procedure for the synthesis of fluoro-substituted chalcones; rather than the conventional methods which presented longer reactions times and poorer yields. The spectroscopic data are in accordance with the expected structures and previous reports in the literature.
ACKNOWLEDGEMENTS
- The authors wish to thank UJAT for the financial support via the project PFICA UJAT-2013-IB-13 and the Centro de Química of the BUAP for the support in obtaining the NMR spectra.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML