-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2016; 6(3): 86-92
doi:10.5923/j.ajoc.20160603.02

Assembled Nanoparticle Films with Crown Ether Derivatives as Sensors for Metal Ions
Kevin W. Kittredge1, Justin T. Malinowski1, Alison M. Washington1, Robert W. Dayand2, Michael C. Leopold2
1Department of Chemistry, Virginia Wesleyan College, Wesleyan Dr., Norfolk, VA, USA
2Department of Chemistry, University of Richmond, Richmond, VA, USA
Correspondence to: Kevin W. Kittredge, Department of Chemistry, Virginia Wesleyan College, Wesleyan Dr., Norfolk, VA, USA.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This work represents our ongoing studies of potentially novel sensing materials that utilize the ionophoric capacity of crown ethers attached to metallic nanoparticles by aromatic linkers and assembled as films. These films were better able to detect changes in potassium ion concentration than those previously reported using alkyl linkers. These nanomaterials are small enough to be considered for invivo and remote sensing applications as well as portable measurement devices.
Keywords: Monolayer protected clusters films, Crown ether, Metal ion sensors, Nanoparticles
Cite this paper: Kevin W. Kittredge, Justin T. Malinowski, Alison M. Washington, Robert W. Dayand, Michael C. Leopold, Assembled Nanoparticle Films with Crown Ether Derivatives as Sensors for Metal Ions, American Journal of Organic Chemistry, Vol. 6 No. 3, 2016, pp. 86-92. doi: 10.5923/j.ajoc.20160603.02.
1. Introduction
- Each year in the United States, there are more than 200 million clinical tests performed for metal cations in physiological systems [1]. Potassium and sodium play critical roles in many of the body’s important functions including nerve conduction, muscle activity, acid-base equilibria, and cardiac regulation. Insufficient levels of potassium, a condition known as hypokalemia, can cause muscle weakness, paralysis, and eventually cardiac arrest. Hyperkalemia or elevated levels of potassium can lead to mental confusion, respiratory muscle failure, and cardiac abnormalities. Even small changes in potassium concentration can signal the onset of some of these conditions [2]. Considering potassium’s important role in vital physiological functions, the measurement of potassium concentration is an excellent diagnostic tool for disorders and it is crucial that clinicians can effectively monitor potassium levels, especially during emergencies. Currently, the majority of these measurements are performed using potentiometry with ion selective electrodes (ISEs) that are capable of selectively detecting individual target analytes in a complex matrix such as blood or urine [3].While this method of analysis has proven effective for years, several disadvantages persist. The ISEs themselves have a limited lifetime and are of substantial cost. ISEs are highly susceptible to fouling, resulting in diminished performance, especially at lower concentration levels where they lack adequate sensitivity [4]. More importantly, the manner in which potassium is currently measured is time consuming at a moment when more immediate information would be more beneficial for expedited diagnosis and treatment of patients. The booming research efforts into nanotechnology have afforded a plethora of new materials with unique properties [5]. One of these materials is metallic nanoparticles or colloidal metal whose size and properties are intermediate between molecular and bulk metal [6]. Of particular interest has been the monolayer protected cluster nanoparticle (MPC) [7], which consists of a metallic core with a peripheral layer of stabilizing molecules, commonly alkanethiolates (Figure 1) [8]. The protective layer of molecules prevents coagulation of the metallic cores back to bulk metal and allows them to be easily handled, dissolved in solution, and recollected from solution [7]. MPCs can be easily modified with additional ligands through facile place-exchange reactions where a functionalized ligand (i.e. fluorophore, redox molecule, or simple functional group) can be incorporated into the peripheral layer of the material (Figure 1A→1B) [9]. Their amenity to functionalization, along with their large surface to volume ratio, makes these nanoparticles an attractive material for potential sensing applications.
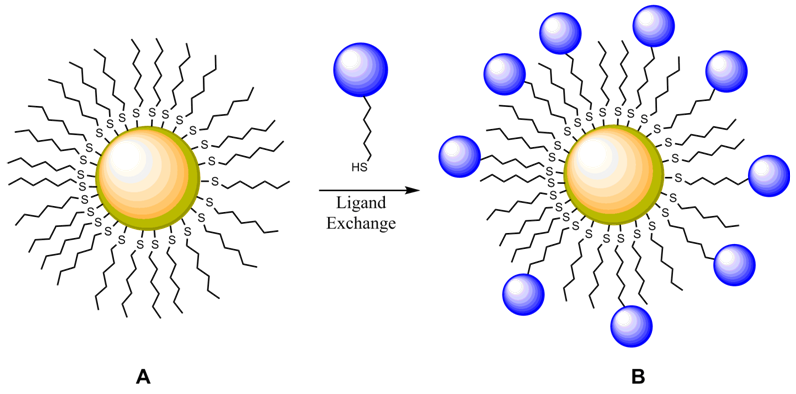 | Figure 1. Monolayer protected cluster (MPC) nanoparticle undergoing ligand exchange |
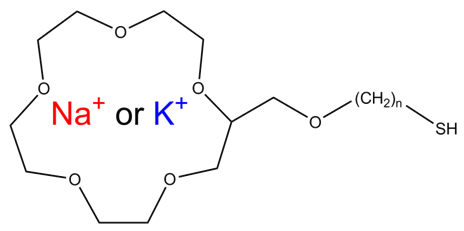 | Figure 2. Structure of alkanethiol modified 15-crown-6 ether |
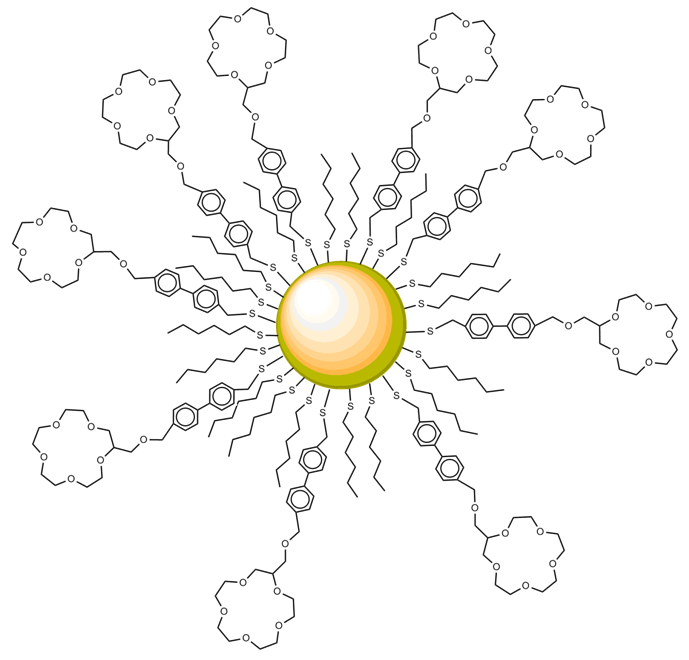 | Figure 3. Structure of biphenyl thiol modified 15-crown-6 ether monolayer protected cluster (CE-MPC) |
2. Experimental
- NaH (Acros, 80% dispersion in mineral oil), THF (Acros, 99.9% anhydrous), 2(hydroxymethyl)15-crown-5 ether (Acros), 4,4’-Bis(bromomethyl)biphenyl (TCI America), tetrabutylammonium fluoride (Acros, 1M in THF containing 5% water), hexamethyldisilathiane (Aldrich), tetrachloroauric(III) acid (Acros), tetranoctylammonium bromide (Avocado), sodium borohydride (Fisher), 1-hexanethiol (Acros), toluene (Aldrich), CH2Cl2 (Acros, extra dry, water <30 ppm, stabilized), and (3mercaptopropyl)-trimethoxysilane (Aldrich) were used as received without further purification. All reactions were run under argon atmosphere. Flash column chromatography was performed with Fisher 60-200 mesh silica gel. All glassware was dried before use at 160°C. 1H and 13C nuclear magnetic resonance spectra were record on aJeol 400 MHz spectrometer in CDCl3 or CD2Cl2.High Resolution mass spectral data (HRMS) were recorded on an Agilent Technologies 6210 LC-TOF by use ofelectron spray ionization (ESI). UV-Vis spectra were recorded on a Perkin Elmer Lambda 35 spectrometer.The synthesis of 2-(4'-Mercaptomethyl-biphenyl-4-methoxymethyl)-15-crown-5 ether (2) is described below and outlined in Scheme 1.
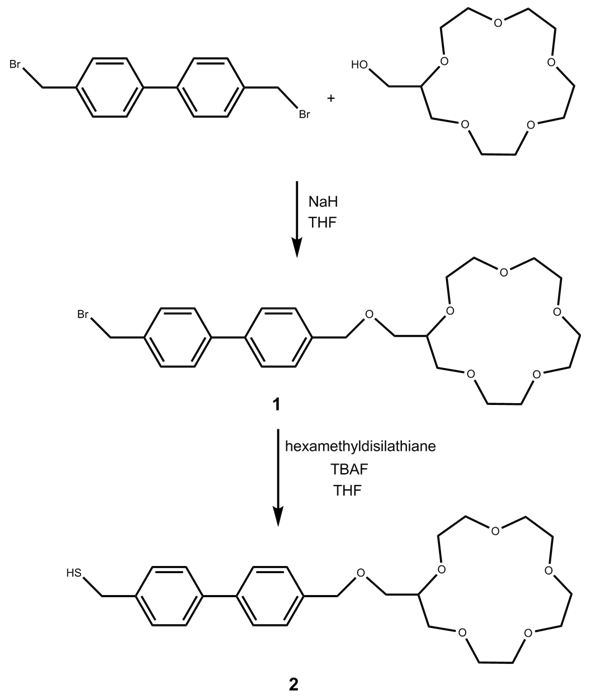 | Scheme 1. Synthesis of 2-(4'-Mercaptomethyl-biphenyl-4 methoxymethyl)-15-crown-5 ether (2) |
3. Results and Discussion
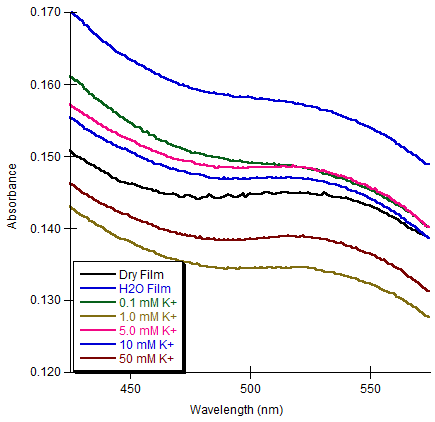
- Film Growth and StructureFor these films to be useful as metal ion sensors the linking and sensing mechanisms must be separate. Also, the films must be quite flexible within their networks so any solvent and ionophores may permeate through the film. Film growth dynamics were monitored spectroscopically, as previously described [12-15, 24]. Films were assembled in dichloromethane, which completely dissolved the CE-MPCs. The biphenyl pendant chain on the crown ether was selected to serve two purposes. First, the two aromatic rings in the biphenyl system would provide a more structurally rigid scaffold upon which the layered film would be constructed, thereby reducing the density of the film upon the addition of subsequent layers. Second, the extended conjugated system would facilitate electronic communication between the coordinated metal in the crown ether and the gold nanoparticle. Our earlier work showed that while electronic communication was occurring, it was seen in only the surface plasmon emission band. This gave very small changes in absorption intensity in the UV-Vis spectrum as a response to metal ion coordination. Figure 4 shows the CEmetal ion – CE bridges that act as the linking mechanism between neighboring nanoparticles. Figure 5 is the absorbance spectra of film growth using sodium ions as the linking metal coordinated in a sandwich configuration. The spectra are typical of MPC films and their growth with a decreasing absorbance at higher wavelengths and a surface plasmon band (SPB) near 540 nm that increases with each exposure to MPCs [19]. Interestingly, the biphenyl linkage produced another absorption in the UV-Vis spectrum at 260 nm. It follows that with each dip cycle, as the film becomes thicker with the immobilization of additional MPC possessing CE ligands. Also, as the film became thicker, a red shift in the absorbance maximum for the SPB was observed (Figure 5, inset), indicative of an increase in MPC cross-linking and a necessary decrease in interparticle spacing [12, 13, 20].
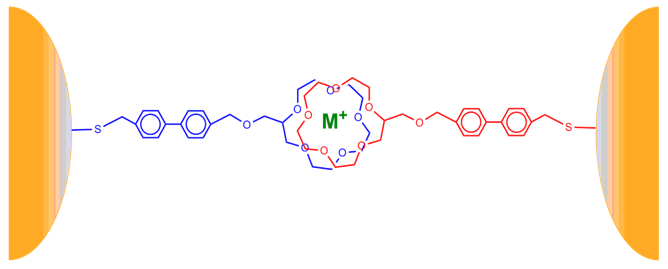 | Figure 4. Structure of CE-MPC metal ion sensing film of biphenyl thiol modified 15 crown-6 ether "sandwich" |
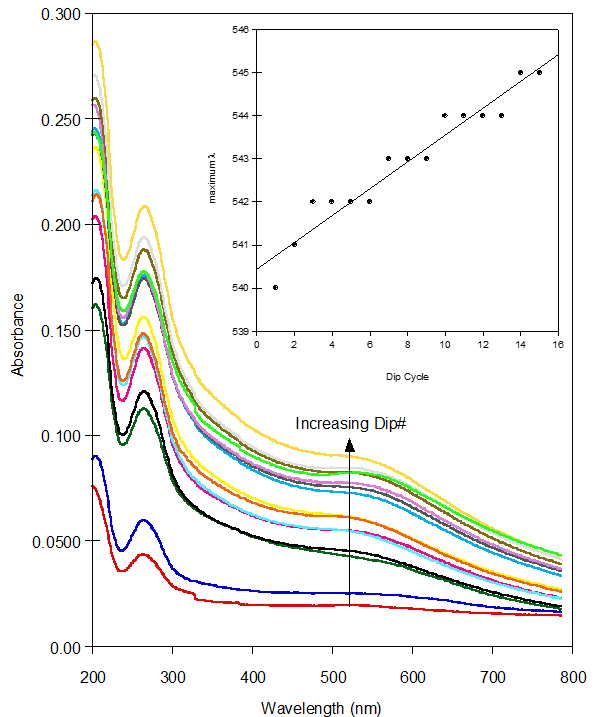 | Figure 5. UV-Vis spectra after each dip cycle during the growth of the CE-MPC. Inset is the plot of maximum λ versus dip cycle. Increasing slope shows red shift as film layers thicken |
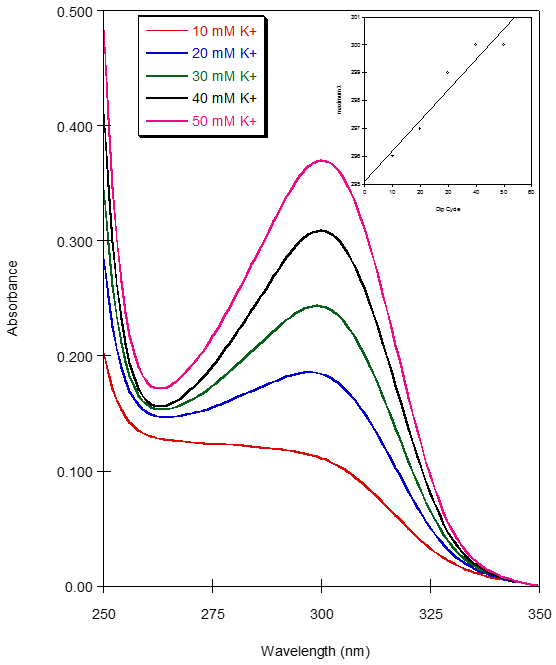
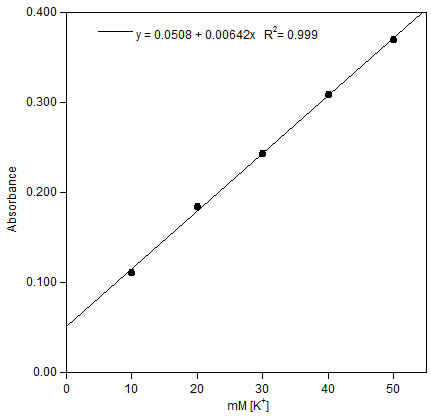 | Figure 8. Plot of Absorbance versus potassium ion concentration for the CE-MPC films. λmax = 300 nm |
4. Conclusions
- The ability to construct a calibration curve demonstrates the utility of these films as diagnostic devices for potassium ion concentration. Future films will be designed containing rigid, aromatic (or polyaromatic) linkers whose purposes will be to strengthen the films structure and control the spacing between layers and help facilitate electronic communication between the metal cation captured in the crown ether and the metallic nanoparticles.
ACKNOWLEDGMENTS
- The investigators gratefully acknowledge the National Science Foundation, CHE-0643709 (JTM & KWK) and CHE-1401593 (MCL) as well as Virginia’s Commonwealth Health Research Board (RWD) for support. KJD was supported by a fellowship from the Virginia Federation of Independent Colleges. Additional support for KWK was provided by a Virginia Wesleyan College Faculty Summer Development Grant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML