-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2016; 6(3): 81-85
doi:10.5923/j.ajoc.20160603.01

Isolation of (-) - Epicatechin from Trichilia emetica Whole Seeds
Abdullahi Usman1, 2, Vera Thoss1, Mohammed Nur-e-Alam1
1School of Chemistry, Bangor University, United Kingdom
2Department of Chemistry, Nasarawa State University Keffi, Nigeria
Correspondence to: Abdullahi Usman, School of Chemistry, Bangor University, United Kingdom.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Trichilia emetica whole seeds were refluxed in water for twenty minutes and the extract subjected to column chromatography and preparative-TLC. The mass spectrum of the isolated compound in positive ion mode showed a parent molecular ion peak at m/z 291 which corresponds to the molecular formula C15H15O6. The structure was established on the basis of nuclear magnetic resonance (NMR), and Infrared (IR) spectroscopy, ultraviolet-visible (UV-vis) spectrophotometry as well as by the comparison of the data reported in the literature. It was concluded that the compound isolated is a biologically important polyphenol, (–)- epicatechin.
Keywords: Trichilia emetica, Meliaceae, Epicatechin, Spectroscopy, Chromatography
Cite this paper: Abdullahi Usman, Vera Thoss, Mohammed Nur-e-Alam, Isolation of (-) - Epicatechin from Trichilia emetica Whole Seeds, American Journal of Organic Chemistry, Vol. 6 No. 3, 2016, pp. 81-85. doi: 10.5923/j.ajoc.20160603.01.
Article Outline
1. Introduction
- Trichilia emetica (Vahl), also known as Natal or Woodland Mahogany, belongs to the Meliaceae family. It is an evergreen tree reaching 35 metres in height and is widely distributed in the tropical and sub-tropical regions of Africa. They are propagated by cuttings and regenerate naturally by root suckers, and seeds [1, 2]. In Africa, different traditions use different parts of this plant in treating several diseases and disorders. In South Africa, the decoction of the root and stem bark is used as an emetic and also as a remedy for cold, pneumonia, and intestinal disorder [3]. In Mali, the powdered root mixed with milk is used as a purgative and a poison antidote [4, 5]. In Senegal, the macerated root bark is used for epilepsy and leprosy while the leaves are taken against blennorrhoea. Finally, in Zimbabwe, the bark is used to induce abortion, and in Nigeria the leaves are used for treating syphilis [1, 2, 3]. The extensive traditional uses of this plant have prompted researchers to screen the solvent extracts for a wide range of biological activities, such as anti-plasmodia [6], antimicrobial [7], anti-oxidant [8], anti-inflammatory [9], anti-schistosomal [10], anti-trypanosomal [11], anticonvulsant [12] and anticancer activity [13]. The aim of this study was to isolate bioactive compounds responsible for these activities. This work reports isolation of the subject compound for the first time from the seeds of T. emetica.
2. Materials and Methods
- Optical rotations were measured in methanol solution on a ADP 440+ polarimeter. The melting point was determined by a Stuart instrument and is uncorrected. The 1H and 13C NMR spectra were recorded on a Bruker Avance (400 MHz) spectrometer with internal references of δH 3.31 and δC 49.0 ppm for CD3OD using TMS (Tetramethylsilane) as an internal standard. A Thermo Instruments HPLC system mass spectrometer with an electrospray ionization (ESI) source was used for recording of the mass and UV spectra. Column chromatography (CC) was performed on Fluorochem silica gel (60Å). Thin layer chromatography (TLC) and Preparative thin layer chromatography (PTLC) were conducted on precoated E. Merck TLC silica gel 60 F254 glass plates, and visualization of the compound was done using UV lamp UVL-14 EL hand held 220V 50Hz 4W 254nm white light by UVP.
2.1. Collection of Plant Materials
- T. emetica seeds were collected from Kumasi, Ghana, in February 2013 and identified by botanist Mr Martin A. Arkoh of Kwame Nkruma University of Science and Technology, Kumasi, Ghana. A voucher specimen TBG-2014-1 was deposited at the herbarium of Treborth Botanical Garden Bangor, UK.
2.2. Preparation of the Plant Materials
2.2.1. Extraction
- Seeds (100 g) were refluxed in water (500 ml) at 100°C for 20 minutes and filtered. The filtrate was concentrated to dryness to give 14.23 g dark gummy extract, which was suspended in water. The aqueous extract was sequentially extracted with chloroform and ethyl acetate (EtOAc) at different pH values (pH 3, pH 7 and pH 10), and the pH was adjusted by the addition of 2 M HCl and 2 M NaOH. The TLC and 1H NMR profiles of all the fractions were assessed. The chloroform and aqueous fractions showed poor TLC and 1H NMR profiles and were discarded, while EtOAc fractions at different pH values showed the same major spots under UV lamp and were investigated further.
2.2.2. Isolation
- Purification of the ethyl acetate extract (1.50 g) was carried out by column chromatography in a polarity gradient manner. Hexane and EtOAc were used as the eluents at gradient mixtures from 100% hexane: 0% EtOAc to 0% hexane: 100% EtOAc. This process was followed by extraction with 100% methanol. Forty three (43) fractions were collected, and based on their TLC profiles, were pooled together. Fractions 24-27 (97 mg), obtained with 40% hexane in EtOAc, were combined and evaporated to dryness under vacuum at 40°C. The combined extract was further purified by PTLC. The plate was developed using Hexane – EtOAc – MeOH (5:4:1, v/v). The main band with light purple colour visualized under UV lamp was scraped from the plate and eluted with the same developing solvents, yielding epicatechin (23 mg).
3. Results and Discussion
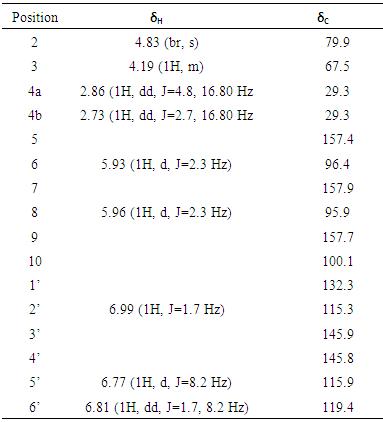
- The compound was obtained as a milky white amorphous powder, with the melting point between 238-239°C and optical rotation of
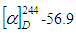 (MeOH: c=0.33), which were comparable to literature values [14]. The compound’s molecular formula of C15H15O6 was established on the basis of ESI-HRMS at m/z 291.0867[M + H]+ (Calcd for 291.0869) (Figure 1). The IR spectrum showed a broad band at 3293 cm-1 region corresponding to phenolic and alcoholic O-H stretching. Other bands at 2910 and 1607 cm-1 were due to saturated C-H stretching and aromatic C=C stretching, respectively. Additionally, the UV spectrum showed absorption peaks at 230 and 279 nm, consistant with conjugated
(MeOH: c=0.33), which were comparable to literature values [14]. The compound’s molecular formula of C15H15O6 was established on the basis of ESI-HRMS at m/z 291.0867[M + H]+ (Calcd for 291.0869) (Figure 1). The IR spectrum showed a broad band at 3293 cm-1 region corresponding to phenolic and alcoholic O-H stretching. Other bands at 2910 and 1607 cm-1 were due to saturated C-H stretching and aromatic C=C stretching, respectively. Additionally, the UV spectrum showed absorption peaks at 230 and 279 nm, consistant with conjugated 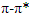 transitions arising from the aromatic rings. These results were similar to those reported in the literature [15].
transitions arising from the aromatic rings. These results were similar to those reported in the literature [15].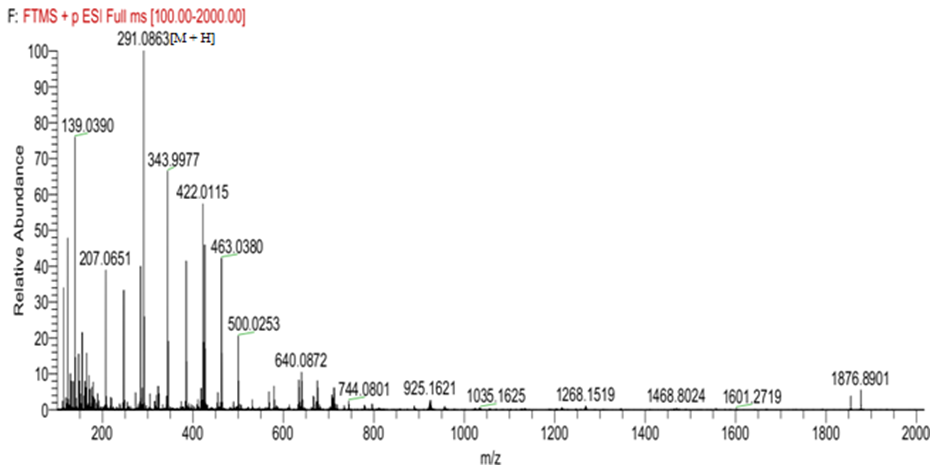 | Figure 1. Mass spectrum of epicatechin |
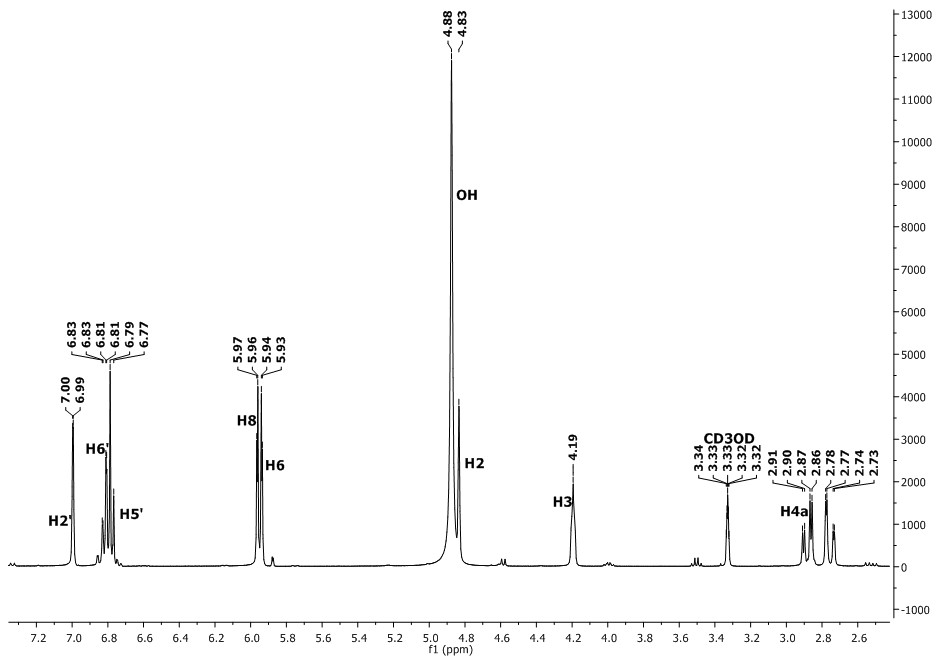 | Figure 2. The  spectrum of epicatechin spectrum of epicatechin |
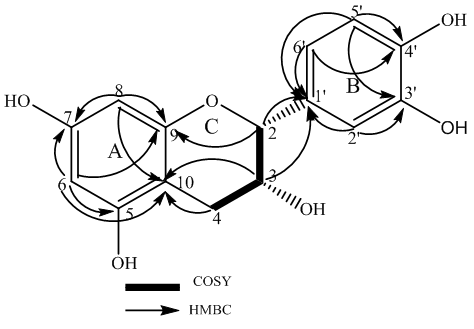 | Figure 3. HMBC and COSY correlations of epicatechin |
|
4. Conclusions
- (-) Epicatechin is a polyphenol with a high antioxidant property and the isolation of this metabolite from T. emetica seems to support some folkloric use of the plant in African traditional medicine. The isolation of the natural product was carried out by using different chromatographic separation and its identity confirmed by polarimetry and spectroscopic techniques.
ACKNOWLEDGEMENTS
- This research work was sponsored by Nigerian Tertiary Education Trust Fund (TETFUND).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML