-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2016; 6(1): 1-7
doi:10.5923/j.ajoc.20160601.01

Synthesis and Antimicrobial Activity of Novel Pyrazole-5-one Containing 1, 3, 4-oxadiazole Sulfonyl Phosphonates
V. Esther Rani , L. K. Ravindranath
Department of Chemistry, S. K. University, Ananthapuramu, India
Correspondence to: V. Esther Rani , Department of Chemistry, S. K. University, Ananthapuramu, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Novel α-aminophosphonate compounds have been prepared in yields ranging from 65%-75% using a Kabachink-Fields (phospha-Mannich) reaction involving the condensation of amine, aldehydes and dialkyl phosphate via schiff base intermediates. The α-aminophosphonates synthesized were tested for their antibacterial and antifungal activity and found to exhibit biological activity. These compounds may serve as potent inhibitors of human and bacterial enzymes, as well as in combating pathological conditions or infections.
Keywords: Antibacterial, Antifungal, 1,3 4-oxadiazole, Phosphonates, 5-Pyrazolone
Cite this paper: V. Esther Rani , L. K. Ravindranath , Synthesis and Antimicrobial Activity of Novel Pyrazole-5-one Containing 1, 3, 4-oxadiazole Sulfonyl Phosphonates, American Journal of Organic Chemistry, Vol. 6 No. 1, 2016, pp. 1-7. doi: 10.5923/j.ajoc.20160601.01.
Article Outline
1. Introduction
- Phosphorus is an element of great importance for living organisms since it is a component of bones and teeth (hydroxyapatite), phospholipids forming cell membranes, DNA and RNA which encode genetic information and an energy carrier–ATP. Despite most known organophosphorus compounds are synthetic. Showing a wide array of applications, they are used as anticorrosive agents, pesticides, herbicides, plasticizers, flame retardants and detergents.Many derivatives 1,3,4-oxadiazole possess derivatives possess a broad range of bioactivity, serving as muscle relaxants [1], analgesic, analgesic, as well as anti inflammatory, anticonvulsive, diuretic, antiemetic properties [2]. These derivatives also have industrial application as phosphosensitizers [3] and liquid crystals [4].5-Pyrazolone derivatives find wide use in medicinal chemistry due to their biological activity. Properties ascribed to this class of compound include anti tuberculosis, anti neoplastic, anti diabetic, anti fertility, anti thyroid and anti microbial activity [5-7].Organophosphorus compounds have been described in the literature as inhibitors of bacteria [5], herbicides, insecticides, pesticides [8, 9], anti-fungal agents [10], anti-HIV [11], anti-cancer [12], anti-viral and anti-inflammatory agents [13]. In view of the above observations, we synthesized several new 5-Pyrazolones incorporating 1,3,4-oxadiazole sulfonyl phosphonates and screened them for possible biological and pharmacological activities. See figure 1.
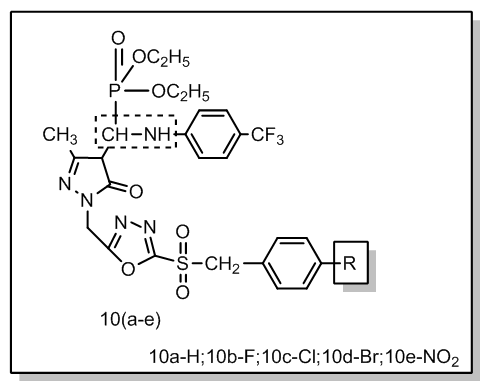 | Figure 1. 5-Pyrazolone 1,3,4-oxadiazole sulfonyl phosphonates prepared in this study |
2. Results and Discussion
- Newly prepared 5-Pyrazolones containing1,3,4-oxadiazole sulfonyl Phosphonates 10(a-e) shown in Scheme-1, were prepared by the reaction of ((1-((5-(benzyl) / (4-fluorobenzyl) / (4-chlorobenzyl) / (4-bromobenzyl) / (4-nitrobenzyl)-1, 3, 4-oxadiazol-2-yl) methyl)-3-methyl-4-(((4-(trifluoromethyl) phenyl) imino) methyl) 1H-pyrazol-5(4H)-one 9(a-e) with diethyl phosphate. The Synthons 9(a-e) were obtained by the reaction of 1-((5-(benzylthio) / ((4-fluorobenzyl)thio) / ((4-chlorobenzyl)thio) / ((4-bromobenzyl)thio) / ((4-nitrobenzyl) thio)-1, 3, 4-oxadiazol-2-yl) methyl)-3-methyl-4-(((4-(trifluoromethyl) phenyl) imino) methyl)-1H-pyrazol-5(4H)-one 8(a-e) with H2O2. The Synthons 8(a-e) were prepared by condensation of 3-Methyl-1-((5-thioxo-4, 5-dihydro-1, 3, 4-oxadiazol-2-yl) methyl)-4-(((4-trifluoromethyl) phenyl) imino) methyl)-1H- pyrazol-5(4H)-one (6) with benzyl chloride / 4-fluoro benzyl chloride / 4-chloro benzyl chloride / 4-bromo benzyl chloride / 4-nitro benzyl chloride 7(a-e). The Synthon (6) was prepared by condensation of 2-(3-methyl-5-oxo-4-(((4- (trifluoromethyl) phenyl) imino) methyl)-4, 5-dihydro- 1H-pyrazol-1-yl) acetohydrazide (5) with KOH and CS2. The Synthon (5) was prepared by condensation of Ethyl 2-(3-methyl-5-oxo-4-(((4-(trifluoromethyl)phenyl) imino) methyl)-4,5-dihydro-1H-pyrazol-1-yl) acetate (4) with hydrazine hydrate. The Synthon (4) was obtained by condensation of 3-Methyl-4-(((4-trifluoromethyl) phenyl) imino) methyl)-1H-pyrazol-5(4H)-one (3) with anhydrous K2CO3, chloroethyl acetate. The Synthon (3) was obtained by condensation of 3-Methyl-5-oxo-4,5-dihydro-1H- pyrazole-4-carbaldehyde (1) with 4-trifluoromethylaniline (2).The structures of the newly synthesized compounds were established by IR, 1H-NMR, 13C-NMR, 31P-NMR and Mass spectral studies.
3. Experimental
3.1. Chemicals and Instrumentation
- The chemicals used in the present investigation were purchased from Sigma-Aldrich Chemicals company, Inc. USA and used without further purification. TLC was performed on aluminum sheet of silica gel 60F254, E-Merk, Germany using iodine as visualizing agent. Melting points were determined in open capillary tubes on Mel-Temp apparatus and are uncorrected. Column chromatography was performed on silica gel with different solvent systems as eluents to afford the pure compound. The IR Spectra were recorded as KBr pellets on Perkin-Elmer 1000 instrument specifications and resolution in cm-1. All 1H and 13C-NMR spectra were recorded on a Varian XL-300 spectrometer operating at 400MHz for 1H-NMR and 75 MHz for 13C-NMR. 31P-NMR spectra were recorded on a Varian XL-spectrometer operating at 161.89MHz. The compounds were dissolved in DMSO-d6 and Drop cap Chemical shifts were referenced to TMS (1H and 13C-NMR) and 85% H3PO4 (31P-NMR). Mass spectral data was recorded on FAB-MS instrument at 70ev with a direct inlet system. Elemental analyses were recorded on a Carlo Erba 1108 elemental analyzer, and were performed by the Central Drug Research Institute, Lucknow, India.
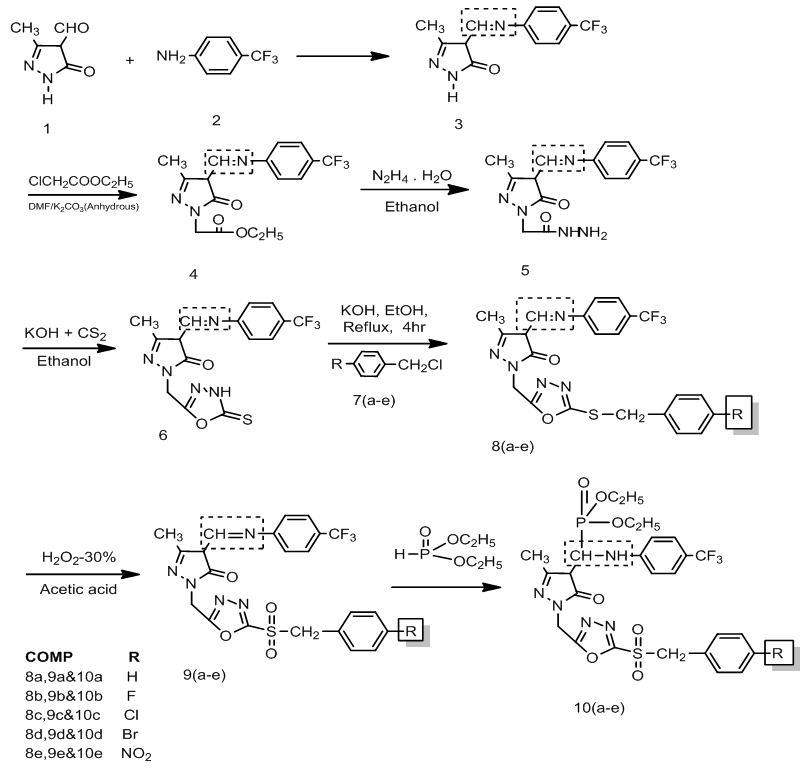 | Scheme 1. Synthesis of Pyrazole-5-one containing 1, 3, 4-oxadiazole sulfonyl Phosphonates 10(a-e) |
3.2. Experimental Procedures
- 3.2.1. 3-Methyl-4-(((4-trifluoromethyl) phenyl) imino) methyl)-1H–pyrazol-5(4H)-one (3)4-trifluoro aniline (8.8gr, 0.05mol) (2) and 3-Methyl-5-oxo-4,5-dihydro-1H-pyrazole-4-carbaldehyde (5.92gr, 0.031mol) (1) were dissolved in absolute alcohol, three drops of acetic acid is added then heated on a steam bath for 5-6 hours at 100°C. After standing for 24 hours at room temperature, the crude product was purified by column chromatography (60-120 mesh silica gel,eluent: 10% EtOAc: Pet Ether) to give the product compound 3-Methyl – 4 - (((4-trifluoromethyl) phenyl) imino) methyl)-1H-pyrazol -5(4H) - one (5.92gr) (3). Yield 70%, m p 156-158°C.3.2.2. Ethyl-2-(3-methyl-5-oxo-4-(((4- (trifluoromethyl) phenyl) imino) methyl)-4,5-dihydro-1H-pyrazol-1-yl) acetate (4)A mixture of 3-Methyl-4-(((4-trifluoromethyl) phenyl) imino) methyl)-1H-pyrazol-5(4H)-one (5.9gr, 0.022mol) (3), anhydrous (4.14gr, 0.06mol) K2CO3, chloroethyl acetate (1.35gr, 0.06mol) and DMF was stirred at room temperature for 8 hours. The reaction mixture was diluted with ice cold water, the crude product was purified by column chromatography (60-120 mesh silica gel,eluent: 10% EtOAc:Pet ether). The solid was identified as Ethyl 2-(3-methyl-5-oxo-4-(((4-(trifluoromethyl) phenyl) imino) methyl)-4, 5-dihydro-1H-pyrazol-1-yl) acetate (5.471gr) (4). Yield 70%, m p 135-137°C.3.2.3. 2-(3–methyl–5–oxo–4-(((4- (trifluoromethyl) phenyl) imino) methyl)-4,5-dihydro-1H-pyrazol-1-yl) acetohydrazide (5)A solution of Ethyl 2-(3-methyl-5-oxo-4-(((4- (trifluoromethyl) phenyl) imino) methyl)-4,5-dihydro- 1H-pyrazol-1-yl) acetate (5.4gr, 0.015mol) (4) and hydrazine hydrate (10ml, 0.025mol) in ethanol was refluxed for 5 hours. The reaction mixture was cooled and poured on to ice cold water with stirring. The separated solid was filtered, washed with water, the crude product was purified by column chromatography (60-120 mesh silica gel,eluent: 10% EtOAc:Pet ether). The solid was identified as 2-(3-methyl-5-oxo-4-(((4-(trifluoromethyl) phenyl) imino) methyl)-4, 5-dihydro-1H-pyrazol-1-yl) acetohydrazide (5.119gr) (5). Yield 75%, m p 163-165°C.3.2.4. 3-Methyl-1-((5-thioxo-4,5-dihydro-1,3,4-oxadiazol- 2-yl)methyl)-4-(((4-trifluoromethyl) phenyl) imino) methyl)-1H-pyrazol-5(4H)-one (6)The condensation of 2-(3-methyl-5-oxo-4-(((4- (trifluoromethyl) phenyl) imino) methyl) - 4, 5 – dihydro - 1H – pyrazol – 1 - yl) acetohydrazide (5.1gr, 0.015mol) (5) with a mixture of 1.68gr, 0.03mol) KOH, ethanol and (3.4gr, 0.04mol) carbon disulphide afforded the corresponding crude product was purified by column chromatography (60-120 mesh silica gel,eluent: 10% EtOAc:Pet ether). The solid was identified as 3-Methyl-1-((5-thioxo-4, 5-dihydro-1, 3, 4-oxadiazol-2-yl) methyl)-4-(((4-trifluoromethyl) phenyl) imino) methyl) - 1H – pyrazol - 5(4H) - one (4.025gr) (6). Yield 70%, m p 140-142°C.3.2.5. 1-((5-(benzylthio) / ((4-fluorobenzyl) thio) / ((4-chlorobenzyl) thio) / ((4-bromobenzyl) thio) / ((4-nitrobenzyl) thio)-1,3,4-oxadiazol-2-yl) methyl)–3–methyl–4- (((4-(trifluoromethyl) phenyl) imino) methyl) -1H–pyrazol-5(4H)-one 8(a-e)To a stirred solution of KOH in ethanol, 3-Methyl-1- ((5-thioxo-4, 5-dihydro-1, 3, 4-oxadiazol-2-yl) methyl)-4- (((4-trifluoromethyl) phenyl) imino) methyl) -1H- pyrazol -5(4H) – one (3.0gr, 0.008mol) (6) and benzyl chloride (1.39gr, 0.01mol) (7a) was added and the reaction mixture was heated to reflux for 4 hours. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature, diluted with water, brine and dried over anhydrous sodium sulphate and the solvent was removed under pressure to get crude 1-((5-(benzylthio)-1, 3, 4-oxadiazol-2-yl) methyl)-3-methyl- 4-(((4-(trifluoromethyl) phenyl) imino) methyl)-1H-pyrazol- 5(4H)-one (8a). The crude (8a) was recrystallized in ethyl acetate and petroleum ether solvent mixture to obtain the pure (2.84gr) (8a). Yield 75% and m p 166-168°C.The similar procedure was adopted to synthesize 8(b-e) (2.55gr-8b;2.76gr-8c;2.70gr-8d; 2.90gr-8e) by condensation of (3.0gr, 0.008mol) (6) with 4-fluoro benzyl chloride (1.73gr, 0.012mol) (7b), 4-chloro benzyl chloride (1.93gr, 0.012mol) (7c), 4-bromo benzyl chloride (2.26gr, 0.01mol) (7d) and 4-nitro benzyl chloride (1.88gr, 0.01mol) (7e) respectively.3.2.6. ((1-((5-(benzyl sulfonyl) / ((4-fluorobenzyl) sulfonyl) / ((4-chlorobenzyl) sulfonyl) / ((4-bromobenzyl) sulfonyl) / ((4-nitrobenzyl) sulfonyl)-1,3,4-oxadiazol-2-yl) methyl) – 3 – methyl – 4 - (((4-(trifluoromethyl) phenyl) imino) methyl)-1H - pyrazol 5-(4H)-one 9(a-e)To a solution of 1-((5-(benzylthio) - 1, 3, 4 – oxadiazol – 2 - yl) methyl) – 3 – methyl – 4 -(((4 - (trifluoromethyl) phenyl) imino) methyl) - 1H–pyrazol - 5(4H) - one (2.36gr, 0.005mol) (8a) in (3ml, 0.05mol) glacial acetic acid, (3ml) 30% hydrogen peroxide was added and the reaction mixture was refluxed for 2 hours. The progress of the reaction was monitored by TLC on silica gel using petroleum ether-ethyl acetate (1:2 v/v) as mobile phase. After completion of the reaction, the solvent was removed by rotary evaporation and the resulting residue was purified by column chromatography on silica gel (100-200 mesh) and ethyl acetate:hexane, (3:7 ratio) as an eluent to afford pure product 1-((5-(benzylsulfonyl) - 1, 3, 4 – oxadiazol – 2 - yl) methyl) – 3 – methyl – 4 - (((4-(trifluoromethyl) phenyl) imino) methyl) - 1H – pyrazol - 5(4H) - one (9a). The final pure product (1.769gr) 9(a) was separated by filtration. The Yield 70%, m p 134-136°C.The similar procedure was adopted to synthesize 9(b-e) (1.70gr-9b, 1.88gr-9c, 1.63gr-9d, 1.78gr-9e) from 8(b-e) (2.45gr, 0.005mol-8b; 2.53gr, 0.005mol-8c; 2.20gr, 0.004mol-8d; 2.59gr, 0.005mol-8e) and (3ml, 30%) H2O2.3.2.7. Diethyl ((1-((5-(benzylsulfonyl) / ((4-fluorobenzyl) sulfonyl) / ((4-chorobenzyl) sulfonyl) / ((4-bromobenzyl) sulfonyl) / ((4-nitrobenzyl) sulfonyl)-1,3,4–oxadiazol–2-yl) methyl)–3– methyl–5–oxo-4,5–dihydro-1H– pyrazol-4-yl)((4-(trifluoromethyl) phenyl) amino) methyl) phosphonate 10(a-e)A mixture of 1-((5-(benzyl sulfonyl) - 1, 3, 4 – oxadiazol – 2 - yl) methyl) – 3 – methyl – 4 - (((4-(trifluoromethyl) phenyl) imino) methyl) - 1H – pyrazol - 5(4H) - one (9a). The final pure product 1.51gr, 0.003mol) 9(a) and Diethyl phosphite (1.2ml, 0.009 mol) in anhydrous toluene (15ml) was added drop wise. Stirring was continued at room temperature for another 0.5 hour, after which the mixture was heated under reflux for 6 hours. The reaction was monitored by TLC on silica gel using petroleum ether-ethyl acetate (1:2 v/v) as mobile phase. After completion of the reaction, the solvent was removed by rotary evaporation and the resulting residue was purified by column chromatography on silica gel (100-200 mesh) and ethyl acetate:hexane, (3:7 ratio) as an eluent to afford pure Diethyl ((1-((5-(benzylsulfonyl) - 1, 3, 4-oxadiazol-2-yl) methyl) – 3 – methyl – 5 – oxo - 4, 5 – dihydro - 1H – pyrazol – 4 - yl) ((4 - (trifluoromethyl) phenyl) amino) methyl) phosphonate (1.35gr) 10(a). Yield 65%, m p 116-118°C.The similar procedure was adapted to synthesized 10(b-e) (1.25gr-10b; 1.42gr-10c; 1.47gr-10d; 1.30gr-10e) by the reaction between 9(b-e) (1.57gr, 0.003mol-9b; 1.61gr, 0.003mol-9c; 1.75gr, 0.003mol-9d; 1.65gr, 0.003mol-9e) with Diethyl phosphite (1.2ml, 0.009 mol) in anhydrous toluene (15ml).
4. Compound Characterization
- The structures of these newly synthesized compounds of 10(a-e) were established by IR, 1H-NMR, 13C-NMR, 31P-NMR, Mass data and elemental analysis. 1. Diethyl ((1-((5-(benzylsulfonyl)-1,3,4-oxadiazol-2-yl) methyl)-3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-4-yl) ((4-(trifluoromethyl) phenyl) amino) methyl) phosphonate 10 (a) according to procedure 3.2.7 to afford the target compound as a yellow solid (1.35gr) with the following characteristic: Yield 70%, m p 116-168°C. IR (KBr 4000-400 cm-1): 3418-3384 (N-H), 3052 (stretching of Ar-H), 2940 and 2895 (CH3 and CH2 of aliphatic-CH), 1670 (C=O), 1615 (N-N), 1478-1375 (stretching vibrations of pyrazole ring), 1315 & 1181 (SO2 str), 1245 (P=O), 1140 (C-O), 1100 (C-F) and 1019 cm-1 (P-O-C(aliphatic)). 1H-NMR (400MHz, DMSO-d6): δ PPM 1.29 (t, 6H, (CH3)2 of diethyl phosphate, J=7.5Hz), 1.94 (s, 3H, of CH3 group), 2.7 (d, 1H, CH of pyrazole ring), 2.9 (d, 1H, CH of methyl phosphonate, J=7.5Hz), 4.0 (d, 1H, NH of (trifluoromethyl)phenyl amine), 4.07 (q, 4H, (CH2)2 of diethyl phosphate, J=7.5Hz), 4.46 (s, 2H of N-CH2), 5.17 (s, 2H of S-CH2), 6.53-7.39 (m, 5H of benzylsulfonyl group) and 7.26-7.40 (m, 4H, CH of trifluoromethylphenyl). 13C-NMR (75 MHz, DMSO-d6): δPPM. 155.6, 33.7, 172.0, 19.3, 57.5, 62.2, 16.3, 70.3, 53.2, 66.4, 150.9, 113.8, 125.9, 124.9 and 124.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11& C14, C12 & C13, C15, C16 & C20, C17 & C19, C18 and C21 respectively. 31P-NMR (161.89 MHz, DMSO-d6): δPPM. 19.50. m/z = 643.15 for C26H29F3N5O7PS. Anal. Found (Calcd) C: 47.72 (48.52), H: 4.04 (3.11), F: 8.06 (10.35), N: 10.28 (15.27), P: 4.11 (4.81), S: 4.78 (5.83).2. Diethyl ((1-((5-((4-fluorobenzyl) sulfonyl)-1,3,4- oxadiazol-2-yl) methyl)-3-methyl-5-oxo-4, 5-dihydro-1H- pyrazol-4-yl) ((4-(trifluoromethyl) phenyl) amino) methyl) phosphonate 10 (b) according to procedure 3.2.7 to afford the target compound as a yellow solid (1.25gr) with the following characteristic: Yield 65%, mp135-137°C. IR (KBr 4000-400 cm-1): 3420-3386 (N-H), 3065 (stretching of Ar-H), 2940 and 2895 (CH3 and CH2 of aliphatic-CH), 1670 (C=O), 1615 (N-N), 1478-1375 (stretching vibrations of pyrazole ring), 1320 & 1185 (SO2 str), 1245 (P=O), 1140 (C-O), 1100 (C-F) and 1025 cm-1 (P-O-C(aliphatic)). 1H-NMR (400MHz, DMSO-d6): δ PPM 1.29 (t, 6H, (CH3)2 of diethyl phosphate, J=7.5Hz), 1.94 (s, 3H, of CH3 group), 2.7 (d, 1H, CH of pyrazole ring), 2.9 (d, 1H, CH of methyl phosphonate, J=7.5Hz), 4.0 (d, 1H, NH of (trifluoromethyl) phenyl amine), 4.07 (q, 4H, (CH2)2 of diethyl phosphate, J=7.5Hz), 4.96 (s, 2H of N-CH2), 5.17 (s, 2H of S-CH2), 7.12-7.40 (m, 4H of fluorobenzylsulfonyl group) and 7.26-7.62 (m, 4H, CH of trifluoromethylphenyl). 13C-NMR (75 MHz, DMSO-d6): δPPM. 155.6, 34.0, 172.2, 19.3, 57.5, 62.2, 16.3, 51.0, 163.2, 166.4, 68.2, 124.7, 130.6, 115.4, 159.9, 150.9, 113.8, 125.9, 124.9 and 124.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11, C12, C13, C14, C15 & C19, C16 & C18, C17, C20, C21 & C25, C22 & C24, C23 and C26 respectively. 31P-NMR (161.89 MHz, DMSO-d6): δPPM. 21.0. m/z = 661.14 for C26H28F4N5O7PS. Anal. Found (Calcd) C: 46.40 (47.20), H: 3.77 (4.27), F: 10.69 (11.49), N: 9.99 (10.59), P: 3.98 (4.68), S: 4.65(4.85).3. Diethyl ((1-((5-((4-chlorobenzyl) sulfonyl)-1,3,4– oxadiazol–2-yl) methyl)–3–methyl–5–oxo-4,5–dihydro– 1H–pyrazol–4-yl) ((4(-trifluoromethyl) phenyl) amino) methyl) phosphonate 10 (c) according to procedure 3.2.7 to afford the target compound as a yellow solid (1.42gr) with the following characteristic: Yield 70%, m p 156-158°C. IR (KBr 4000-400 cm-1): 3420-3390 (N-H), 3055 (stretching of Ar-H), 2940 and 2895 (CH3 and CH2 of aliphatic-CH), 1670 (C=O), 1615 (N-N), 1478-1375 (stretching vibrations of pyrazole ring), 1327 & 1190 (SO2 str), 1259 (P=O), 1140 (C-O), 1100 (C-F), 1020 (P-O-C(aliphatic)) and 730 cm-1(C-Cl). 1H-NMR (400MHz, DMSO-d6): δ PPM 1.29 (t, 6H, (CH3)2 of diethyl phosphate, J=7.5Hz), 1.94 (s, 3H, of CH3 group), 2.7 (d, 1H, CH of pyrazole ring), 2.9 (q, 1H, CH of methyl phosphonate, J=7.5Hz), 4.0 (d, 1H, NH of (trifluoromethyl)phenyl amine), 4.07 (q, 4H, (CH2)2 of diethyl phosphate, J=7.5Hz), 4.96 (s, 2H of N-CH2), 5.17 (s, 2H of S-CH2), 7.16-7.39 (m, 4H, CH of trifluoromethylphenyl) and 7.34-7.47 (m, 4H of chlorobenzylsulfonyl group). 13C-NMR (75 MHz, DMSO-d 6): δPPM. 155.6, 34.0, 172.2, 19.3, 57.5, 62.2, 16.3, 51.0, 163.2, 166.4, 68.2, 127.2, 130.4, 128.7, 131.3, 150.9, 113.8, 125.9, 124.9 and 124.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11, C12, C13, C14, C15 & C19, C16 & C18, C17, C20, C21 & C25, C22 & C24, C23 and C26 respectively. 31P-NMR (161.89 MHz, DMSO-d6): δPPM. 20.50. m/z = 677.11 for C26H28ClF3N5O7PS. Anal. Found (Calcd) C: 45.26 (46.06), H: 3.66 (4.16), Cl: 4.43 (5.23), F: 7.61 (8.41), N: 9.63 (10.33), P: 3.87 (4.57), S: 4.53 (4.73).4. Diethyl ((1-((5-((4-bromobenzyl) sulfonyl)-1,3 4 oxadiazol–2-yl) methyl)–3–methyl–5–oxo-4,5–dihydro- 1H–pyrazol–4-yl) ((4-(trifluoromethyl) phenyl) amino) methyl) phosphonate 10 (d) according to procedure 3.2.7 to afford the target compound as a yellow solid (1.47gr) with the following characteristic: Yield 68%, m p 168-170°C. IR (KBr 4000-400 cm-1): 3422-3392 (N-H), 3060 (stretching of Ar-H), 2940 and 2895 (CH3 and CH2 of aliphatic-CH), 1670 (C=O), 1615 (N-N), 1478-1375 (stretching vibrations of pyrazole ring), 1310 & 1178 (SO2 str), 1256 (P=O), 1140 (C-O), 1100 (C-F), 1029 (P-O-C(aliphatic)) and 650 cm-1(C-Cl). 1H-NMR (400MHz, DMSO-d6): δ PPM 1.29 (t, 6H, (CH3)2 of diethyl phosphate, J=7.5Hz), 1.94 (s, 3H, of CH3 group), 2.7 (d, 1H, CH of pyrazole ring), 2.9 (q, 1H, CH of methyl phosphonate, J=7.5Hz), 4.0 (d, 1H, NH of (trifluoromethyl) phenyl amine), 4.07 (q, 4H, (CH2)2 of diethyl phosphate, J=7.5Hz), 4.96 (s, 2H of N-CH2), 5.17 (s, 2H of S-CH2), 7.16-7.40 (m, 4H, CH of trifluoromethylphenyl) and 7.31-7.85 (m, 4H of bromobenzylsulfonyl group). 13C-NMR (75 MHz, DMSO-d6): δPPM. 155.6, 34.0, 172.2, 19.3, 57.5, 62.2, 16.3, 51.0, 163.2, 166.4, 68.2, 128.1, 131.2, 131.5, 120.1, 150.9, 113.8, 125.9, 124.9 and 124.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11, C12, C13, C14, C15 & C19, C16 & C18, C17, C20, C21 & C25, C22 & C24, C23 and C26 respectively. 31P-NMR (161.89 MHz, DMSO-d6): δPPM. 19.70. m/z = 723.06 for C26H28BrF3N5O7PS. Anal. Found (Calcd) C: 42.42 (43.22), H: 3.61 (3.91), Br: 10.4 (11.06), F: 7.09 (7.89), N: 9.09 (9.69), P: 3.69 (4.29); S: 4.24 (4.44).5. Diethyl ((3–methyl–1-((5-((4-nitrobenzyl) sulfonyl)-1, 3,4-oxadiazol-2-yl) methyl)–5–oxo-4,5–dihydro-1H – pyrazol–4-yl) ((4-(trifluoromethyl) phenyl) amino) methyl) phosphonate 10 (e) according to general procedure 3.2.7 to afford the target compound as a yellow solid (1.30gr) with the following characteristic: Yield 65%, m p 143-145°C. IR (KBr 4000-400 cm-1): 3418-3384 (N-H), 3067 (stretching of Ar-H), 2940 and 2895 (CH3 and CH2 of aliphatic-CH), 1670 (C=O), 1615 (N-N), 1478-1375 (stretching vibrations of pyrazole ring), 1318 & 1180 (SO2 str), 1254 (P=O), 1140 (C-O), 1100 (C-F) and 1010 cm-1 (P-O-C(aliphatic)). 1H-NMR (400MHz, DMSO-d6): δ PPM 1.29 (t, 6H, (CH3)2 of diethyl phosphate, J=7.5Hz), 1.94 (s, 3H, of CH3 group), 2.7 (d, 1H, CH of pyrazole ring), 2.9 (q, 1H, CH of methyl phosphonate, J=7.5Hz), 4.0 (d, 1H, NH of (trifluoromethyl) phenyl amine), 4.07 (q, 4H, (CH2)2 of diethyl phosphate, J=7.5Hz), 4.96 (s, 2H of N-CH2), 5.17 (s, 2H of S-CH2), 7.16-7.40 (m, 4H, CH of trifluoromethylphenyl) and 7.49-7.60 (m, 4H of nitrobenzylsulfonyl group). 13C-NMR (75 MHz, DMSO-d6): δPPM. 155.6, 34.0, 172.2, 19.3, 57.5, 62.2, 16.3, 51.0, 163.2, 166.4, 68.2, 135.2, 129.9, 123.8, 144.9, 150.9, 113.8, 125.9, 124.9 and 124.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11, C12, C13, C14, C15 & C19, C16 & C18, C17, C20, C21 & C25, C22 & C24, C23 and C26 respectively. 31P-NMR (161.89 MHz, DMSO-d6): δPPM. 22.75. m/z = 688.13 for C26H28F3N6O9PS. Anal. Found (Calcd) C: 44.55 (45.35), H: 3.60 (4.10), F: 7.48 (8.28), N: 11.61 (12.21), P: 3.80 (4.50), S: 4.46 (4.66).
5. Biological Activity
- The antimicrobial activity [14] of these newly synthesized compounds was performed according to disc diffusion method, as recommended by the National Committee for Clinical Laboratory. The synthesized compounds were used at the concentration of 250μg/ml DMF as a solvent [15].
5.1. Anti-Bacterial Activity
- The antibacterial activity of 5-Pyrazolone containing 1,3,4-oxadiazole sulfonyl Phosphonates 10(a-e) were screened against the Staphylococcus aureus and Bacillus cerus (gram positive) and Escherichia coli, Pseudomonasaeruginosa (gram negative) organisms. Here Amoxicillin is tested as reference compound to compare the activity.
5.2. Anti-Fungal Activity
- The anti-fungal activity of 5-Pyrazolone containing 1,3,4-oxadiazole sulfonyl Phosphonates 10(a-e) was screened against the Aspergillus niger and Candida albicans. Here Ketoconazole is tested as reference compound to compare the activity.The compounds of nitro (10e) and chloro (10c) substituted may be exhibit good antibacterial activity against both bacteria and fungi. The electronegativity groups NO2, Cl substituents containing compounds were shows high biological activity.The antibacterial and anti-fungal activity was shown in the Table 1 and depicted in Figure 2.
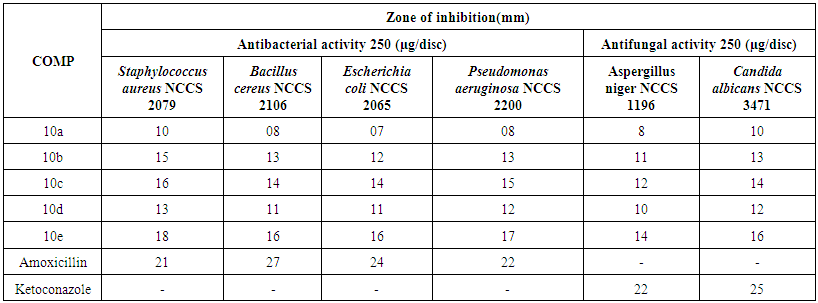 | Table 1. Antibacterial and anti-fungal activity (Diameter zone of Inhibition in mm) of Compounds of 10(a-e) (250 µg/ml) |
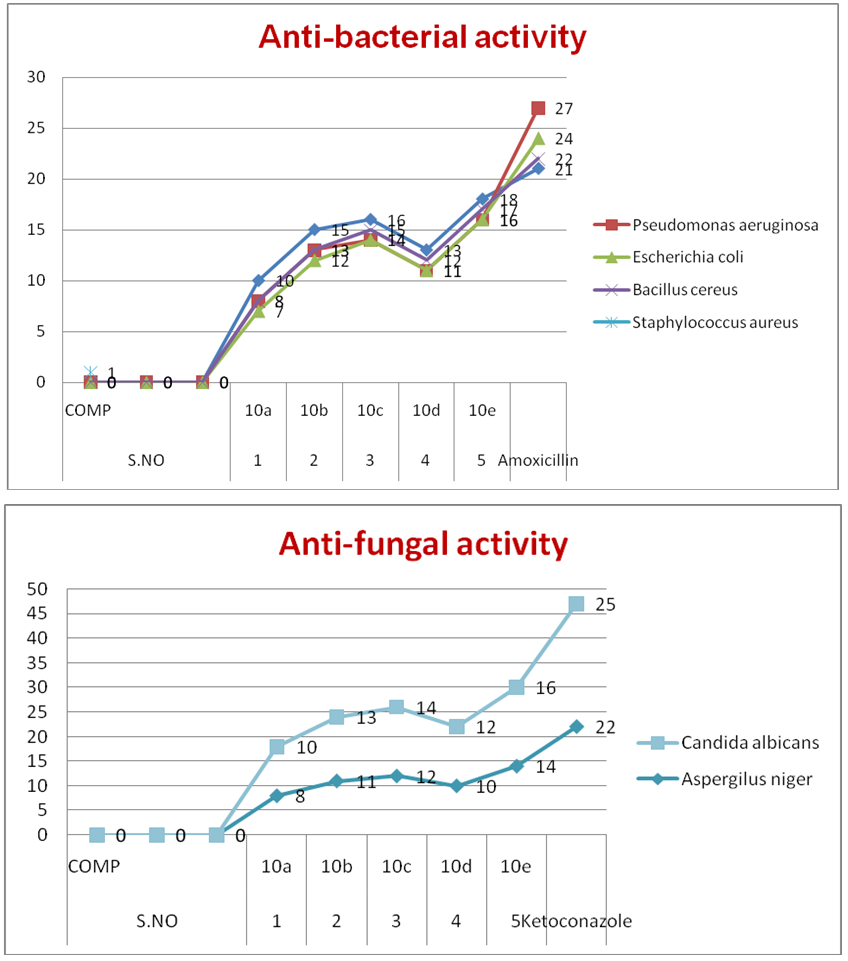 | Figure 2. Biological activities for Compounds 10(a-e) |
6. Conclusions
- In conclusion, we have demonstrated a successful 7-step synthesis of 5-Pyrazolone containing 1,3,4-oxadiazole sulfonyl Phosphonates moieties. These new examples have been shown to have both antibacterial and antifungal activity and may serve as pharmacological agents.
ACKNOWLEDGEMENTS
- The author V.E.R thanks U G C – S A P and U G C – B S R, New Delhi for financial assistance. The authors are also thankful to IICT Hyderabad and CDRI Lucknow for spectral and analytical data.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML