-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2015; 5(4): 113-115
doi:10.5923/j.ajoc.20150504.01
The Microwave-Assisted Solventless Reduction of Aldehydes and Ketones Using Silica Supported Sodium Borohydride
Rachel A. Courtney, Stephen S. McCarron, Renee A. Fowble, Lori L. White, Kevin W. Kittredge
Department of Chemistry, Virginia Wesleyan College, United States of America
Correspondence to: Renee A. Fowble, Lori L. White, Kevin W. Kittredge, Department of Chemistry, Virginia Wesleyan College, United States of America.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The rapid, solventless reduction of aldehydes and ketones is described. Using sodium borohydride supported in silica gel and microwave irradiation gave the corresponding alcohols in high yields, with reduced reaction time and less waste compared to the conventional reductions. The percent conversion was dependent on the steric hindrance within the carbonyl compound and not directly related to the charge on the carbonyl carbon.
Keywords: Solid-State synthesis, Microwave-Assisted, Reductions, Solventless
Cite this paper: Rachel A. Courtney, Stephen S. McCarron, Renee A. Fowble, Lori L. White, Kevin W. Kittredge, The Microwave-Assisted Solventless Reduction of Aldehydes and Ketones Using Silica Supported Sodium Borohydride, American Journal of Organic Chemistry, Vol. 5 No. 4, 2015, pp. 113-115. doi: 10.5923/j.ajoc.20150504.01.
Article Outline
1. Introduction
- We are reporting the microwave-assisted reduction of various carbonyl compounds using solid-state supported sodium borohydride. This reaction method is a possible alternative and complementary to the traditional reduction using metal borohydrides in solution. The reduction of carbonyl groups by sodium borohydride is a common reaction in organic chemistry. [1] Sodium borohydride is a relatively mild reducing agent that allows for selectivity of reduction site and more versatile than other non-borohydride reducing agents with regards to solvents and reactants that can be used. [2, 3] The usefulness of sodium borohydride as a reducing agent is generally limited by the reaction time necessary to complete the reductions. Most sodium borohydride reductions take several hours for completion. [2, 3] It was reported that a solvent-free procedure required more than five days for the reduction of a cyclohexanone derivative in modest (54%) yield. [4] Microwave-assisted synthesis has been shown to accelerate organic reactions and may be used as an alternative to conventional heating methods. [1-3, 5] Current theory relates the reaction acceleration to the ability of microwaves to more evenly and rapidly heat reactants and reagents at the molecular level. [5, 6] A wide variety of microwave-assisted reactions have been reported, but the overwhelming majority are performed in solution. [5, 6] Solvent-free techniques employ neat reactants and/or reagents that are supported on a solid matrix. Solid-support techniques require the use of less organic solvents and in some cases, allow for recycling of the support material. Microwave-assisted solvent-free reactions have been shown to both decrease reaction times and increase product yields. [3, 5, 7] Solvent-free reactions can also be conducted in open vessels, will produce less pressure, [2] and are more easily scaled-up for production than solvent-based microwave reactions. [8] These methods are known to decrease the environmental impact of the reaction. [2, 8, 9] There are several examples of the microwave-assisted reduction of ketone and aldehyde compounds by sodium borohydride supported on alumina in the literature. [3, 2, 10, 8, 11] Prior work by our group has previously demonstrated the microwave-assisted reduction of cyclohexanone to cyclohexanol using sodium borohydride supported on a silica matrix. [12] Herein we wish to report the versatility of microwave-assisted solid-supported sodium borohydride reductions for ketone and aldehyde compounds with varying degrees of steric hindrance and a variety of functional groups. (Figure 1)
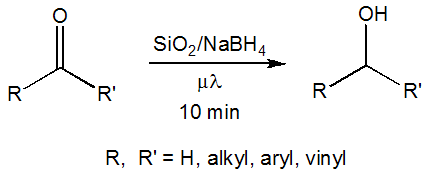 | Figure 1. Reaction scheme for the reduction of carbonyl compounds to corresponding alcohols |
2. Experimental
2.1. Materials and Equipment
- All reactant ketones and aldehydes, diethyl ether, silica gel, and sodium borohydride were purchased from Aldrich Chemical Company and used as received. The reactions were run in 50 mL PTFE-lined, glass microwave reaction vessels and irradiated with a Milestone START microwave labstation. The products were characterized by 1H NMR spectroscopy and GC-MS and compared to authentic samples. GC-MS analysis was conducted on an Agilent 5977A Extractor XL MSD with a chemical ionization detector. Split injections with a 100:1 split ratio was used. The GC was fitted with an HP-5 (5% phenyl: 95% methyl silicone) column (dimensions: 30 m x 0.32 mm; flow rate: 1.64 mL/min; Injector temperature: 250°C; Oven temperature: 150°C; Detector temperature: 290°C). 1H NMR spectra were recorded on a JEOL 400 MHz spectrometer and spectra were obtained in CDCl3. Carbonyl charges were determined using Spartan Student v. 6.1.4 measuring the energy at the ground state with the B3LYP 6-31G* calculation.
2.2. General Procedure
- A mixture of sodium borohydride (200 mg) and silica gel (400 mg) were combined and ground with a mortar and pestle to produce the supported reducing agent. The sodium borohydride/silica mixture was combined with 600 mmol of the aldehyde or ketone in a 50 mL PTFE-lined, glass microwave reaction vessel and mixed thoroughly. The cap was securely fastened, placed in the microwave, and irradiated at 75% power (900 W) for 10 minutes. The alcohol product was extracted from the solid support with 5 mL diethyl ether and gravity filtered through a fritted funnel. The solvent was removed in vacuo to give the alcohol product. Percent conversion was determined by GC and product identification confirmed by 1H-NMR by comparison to an authentic sample.
3. Results and Discussion
- Reaction Yields Different aldehyde and ketone compounds were reduced by sodium borohydride supported in a silica matrix with the assistance of microwave irradiation in excellent yields (Table 1). Percent conversion was greater than 70% for twenty-eight of the thirty ketones and aldehydes studied. The only exceptions were trans-cinnamaldehyde where the carbonyl group is highly conjugated and the sterically hindered carbonyl group of 2,6-dimethyl-4-heptanone. All microwave-assisted reductions proceeded to completion within ten minutes. Allowing the reaction to continue for additional time did not result in noticeable increases in yield. The silica may be washed with methanol followed by water and then dried in an oven to be reloaded with sodium borohydride. Recycled silica showed no difference in its ability to support the reducing agent.
|
4. Conclusions
- We have developed a versatile method for the microwave-assisted reduction of aldehydes and ketones using sodium borohydride. Reactions are completed within ten minutes and typically result in a high percent conversion. The resulting alcohols are easily isolated and the silica support can be refreshed and reused for subsequent reductions.
ACKNOWLEDGEMENTS
- RAC, SSM and KWK gratefully acknowledge the National Science Foundation (CHE0643709) for support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML