-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2015; 5(3): 105-111
doi:10.5923/j.ajoc.20150503.03
A Short-Step Synthesis of syn-1,6:8,13-Dimethano[14]annulene Dicarboximides
Mitsunori Oda 1, Miyako Neha 1, Yoshimitsu Kumai 1, Akira Ohta 1, Ryuta Miyatake 2, Yanmei Zhang 3, Shigeyasu Kuroda 3
1Deptartment of Chemistry, Faculty of Science, Shinshu University, Matsumoto, Nagano, Japan
2Centre for Environmental Conservation and Research Safety, University of Toyama, Toyama, Japan
3Deptartment of Applied Chemistry, Graduate School of Science and Engineering, University of Toyama, Toyama, Japan
Correspondence to: Ryuta Miyatake , Centre for Environmental Conservation and Research Safety, University of Toyama, Toyama, Japan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Double aldol condensation of cyclohepta-1,3,5-triene-1,6-dicarbaldehyde (5) with glutaraldehyde in the presence of piperidine in AcOH furnished bicyclo[5.4.1]dodeca-1(11),2,5,7,9-pentaene-3,5-dicarbaldehyde (12), presenting an alternative shortcut method for preparation of 12. Reaction of dialdehyde 12 with phosphorane reagent 6a in AcOH yielded mono- and double-Wittig adducts, 15 and 16. The mono-adduct 15 undergoes intramolecular cyclization under basic conditions to give the title dicarboximide 10a. Reactions of 12 with phosphorane reagents, 6a and 6b, under basic conditions resulted in direct formation of 10a and 10b, respectively. It is worthy to note that a dimethano[14]annulene derivative can be synthesized in two steps from 5. Results of X-ray diffraction analysis and spectroscopic properties of 10a are also described.
Keywords: [14]Annulene, Bridged annulene, Aldol condensation, Wittig reaction, Dicarboximide
Cite this paper: Mitsunori Oda , Miyako Neha , Yoshimitsu Kumai , Akira Ohta , Ryuta Miyatake , Yanmei Zhang , Shigeyasu Kuroda , A Short-Step Synthesis of syn-1,6:8,13-Dimethano[14]annulene Dicarboximides, American Journal of Organic Chemistry, Vol. 5 No. 3, 2015, pp. 105-111. doi: 10.5923/j.ajoc.20150503.03.
Article Outline
1. Introduction
- We recently reported short-step syntheses of N-substituted 1,6-methno[10]annulene-3,4-dicarboximides (1) and their benzene-, thiophene- and naphthalene-annulated compounds, (2–4). [1] The annulenedicarboximides 1 were obtained in good yields from cyclohepta-1,3,5-triene-1,6- dicarbaldehyde (5) by its condensation with phosphorane reagents 6 in refluxing acetic acid (AcOH), providing an improved synthetic process from 5 to 1 compared with previously reported methods. [2–3] The annulene- dicarboximides annulated by an aromatic ring, 2–4, were obtained from their corresponding annulated dicarbaldehydes 7–9 by their condensation with 6 under basic conditions (Scheme 1). In this paper, we describe an application of this efficient strategy with phosphorane reagents 6 to short-step synthesis of the title compounds 10, novel dicarboximides with a π-extended [14]annulene skeleton bridged by two methylene groups. [4–5] Also, the solid-state structure and spectroscopic properties of 10a are disclosed.
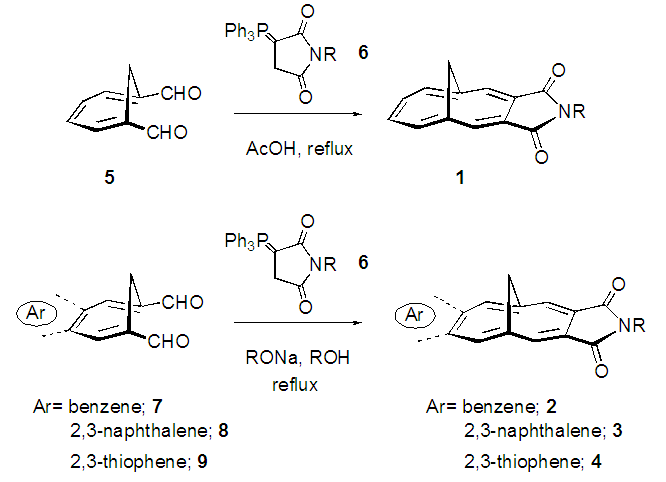 | Scheme 1. Synthetic methods for various 1,6-methano[10]annulene- 3,4-dicarboximides and their aromatic ring-fused compounds |
2. Results and Discussion
2.1. Synthesis of syn-1,6:8,13-dimethano[14]annulene Dicarrboximides
- We planned a synthetic route to the title compounds 10 from 5 via bicyclic dialdehyde 12, as shown in Scheme 2. Vogel et al. reported synthesis of 12 in three steps from 5, involving Horner-Wadsworth-Emmons (HWE) olefination [6] to yield diester 13, subsequent DIBAL (diisobutylaluminium hydride) reduction and final oxidation with DDQ (2,3-dichloro-5,6-dicyano-p-benzoquinone), as shown in Scheme 3. [5] The structure of 12 was confirmed to have syn-stereochemistry between two methylene groups by X-ray crystallographic analysis. [7] In this study, we examined a modified method for preparation of 12.
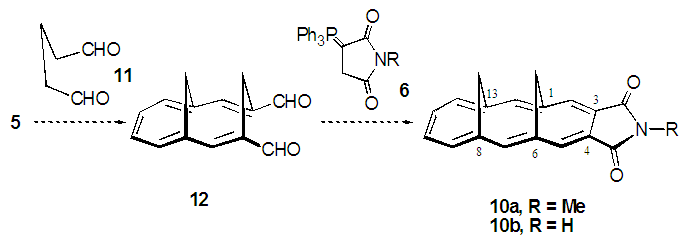 | Scheme 2. A short-step synthetic plan for syn-1,6:8,13-dimethano- [14]annulene-3,4-dicarboximides 10a-b |
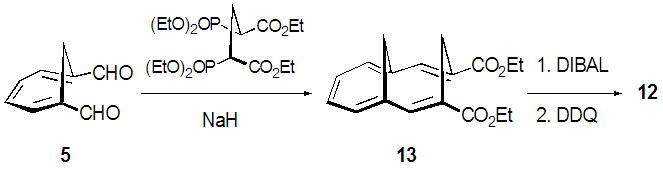 | Scheme 3. A reported synthetic way to 12 via 13 by Vogel et al |
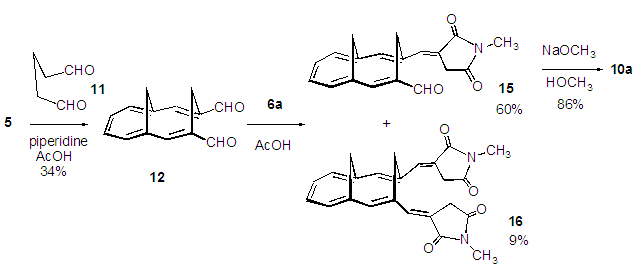 | Scheme 4. Synthesis of 10a via 12 and 15 from 5 |
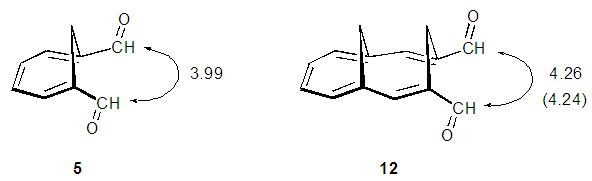 | Figure 1. Calculated distances (Å) between two carbonyl carbon atoms for 5 and 12. A value in a parenthesis is taken from the X-ray structure |
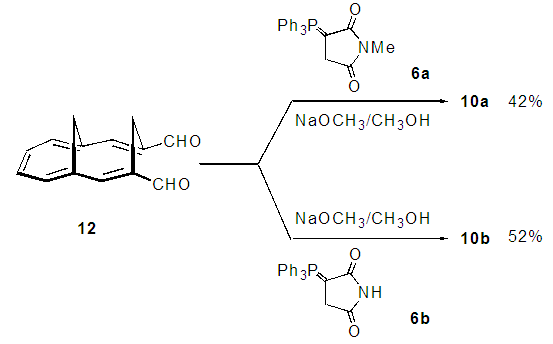 | Scheme 5. One-pot synthesis of 10a-b from 12 |
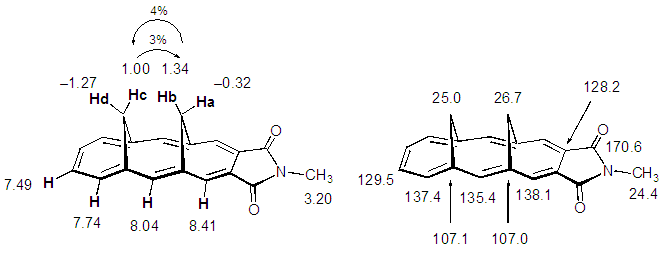 | Figure 2. 1H and 13C signal assignment (δppm) for 10a and results of NOE experiments indicated in % |
2.2. Structure and Properties of syn-1,6:8,13-dimethano- [14]annulene Dicarboximides
- Compounds 10a-b were obtained as orange crystals. Assignment of NMR signals and results of NOE experiments for 10a are shown in Fig. 2. [11] The syn-stereochemistry of two methylene bridges in 10a, expected by the stereochemistry of the starting dialdehyde 12, was confirmed by NOE correlation between the inner hydrogen atoms, Hb and Hc, and also by X-ray diffraction analysis (vide infra). The hydrogen atoms at the methylene bridges were observed shielded by the aromatic [14]annulene ring, but resonate at slightly lower field (δav. = 0.75 ppm) compared with those of the parent hydrocarbon, syn-1,6:8,13- dimethano[14]annulene (17) (δav. = –0.09 ppm). Since the low field shift was observed only for Ha and Hb in 10a, this shift is probably indebted to a deshielding effect of the carbonyl groups in the dicarboximide moiety. The structure of 10b was similarly supported by spectroscopic analyses. The solid-state structure of 10a was determined by X-ray diffraction analysis. ORTEP drawings are shown in Fig. 3. The syn-stereochemistry between two methylene bridges is seen clearly in the side view of ORTEP drawings. Average distance of two inner hydrogen atoms is estimated to be 1.638 Å, which is slightly shorter than that (1.78 Å) in 17. [12] An average length of C–C bonds in the [14]annulene ring of 10a (1.396 Å) and its deviation (±0.028 Å) are very similar to those found in 17 (1.392 Å, ±0.027 Å)[13], indicating that annulation of the imide ring on the [14]annulene ring does not affect the bond-convergency of the [14]annulene ring, as seen in 1. [1]
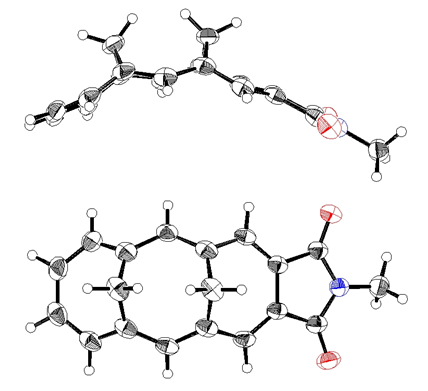 | Figure 3. ORTEP drawings of one of two different molecules of 10a in a cell. A side view (top) and top view (bottom). Oxygen atoms are shown in red and nitrogen atoms in blue |
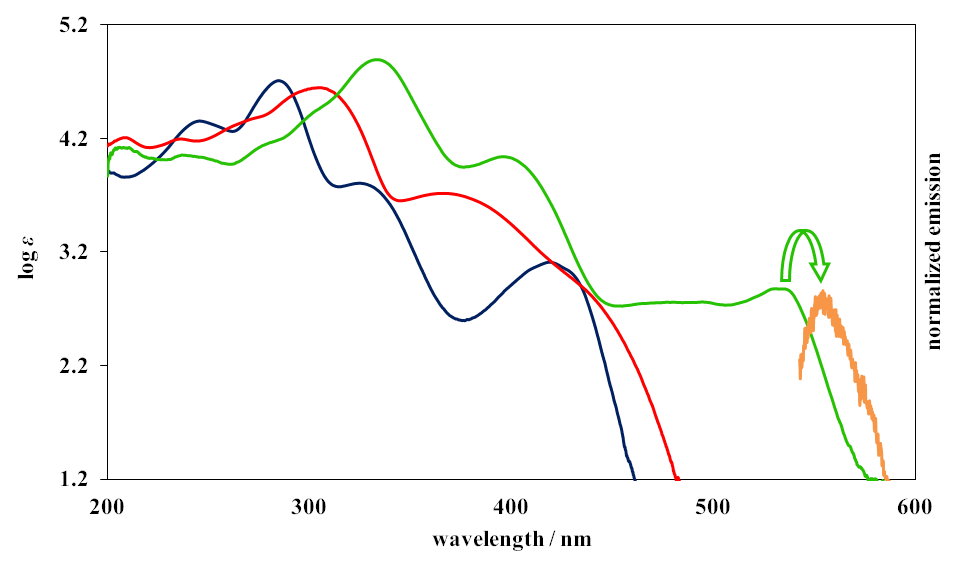 | Figure 4. The UV-vis absorption spectra of 1 (R=Me, blue), 2 (R=Me, red), and 10a (green) in MeOH and the normarized emission spectrum of 10a (orange) |
3. Experimental
3.1. General Remarks
- Melting points were measured on a Yanaco MP-3 and are uncorrected. IR spectra were recorded on a JASCO FT/IR-4100 spectrometer. 1H- and 13C-NMR spectra were recorded on JEOL λ400 and ECA500 spectrometers. Chemical shift values of tetramethylsilane (δ= 0 ppm) for 1H-NMR spectra and CDCl3 (δ= 77.0 ppm) for 13C-NMR spectra were used as internal standard. Mass spectra were measured on a JMS-700 mass spectrometer. Column chromatography was performed with Silica gel 60N from Kanto Chem. Glutaraldehyde (50% aqueous solution) and cyclohepta-1,3,5-triene were purchased from Sigma- Aldrich. Triphenylphosphine, maleimide, and N-methylmaleimide were purchased from Tokyo Chem. Ind. Cyclohepta-1,3, 5-triene-1,6-dicarbaldehyde (5) was prepared from cyclohepta-1,3,5-triene in six steps according to the method of Vogel et al. [5, 16–17] Phosphorane reagents, N-methyl and unsubstituted triphenylphosphonylidenesuccimides, 6a and 6b, were prepared from triphenylphosphine and corresponding maleimides by our reported method. [1, 18]
3.2. Synthesis of bicyclo[5.4.1]dodeca-1(11),2,5,7,9- pentaene-3,5-dicarbaldehyde (12)
- A 50% glutaraldehyde aqueous solution (2.00 g, 10.0 mmol) was added slowly to a refluxing solution of 5 (740 mg, 5.00 mmol) and piperidine (0.987 mL, 10.0 mmol) in 10 mL of AcOH under nitrogen atmosphere. After the addition, the resulted mixture was further refluxed for 4 h. The resulted dark brown reaction mixture was poured into cold water (200 mL) and was extracted with ether/hexane (30 mL x 4). The combined organic layer was washed with a saturated NaHCO3 solution and brine, and was dried over Na2SO4. The solvent was removed under reduced pressure and the residue was purified by silica gel chromatography (ether/hexane =15/85) to give 360 mg (34%) of 12 as yellow prisms. M.p. = 213–215°C (lit. 215–216°C). IR (KBr) νmax =1669 (s), 1655 (s), 1594 (s), 939 (s), 752(s) cm–1; 1H NMR (CDCl3, 400 MHz) δ= 9.36 (s, 2H), 7.15 (m, 4H), 6.66 (s, 2H), 4.98 (d, J = 15.8 Hz , 1H), 4.13 (d, J = 12.2 Hz, 1H), 3.34 (d, J = 15.8 Hz, 1H), 0.66 (d, J = 12.2 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz) δ= 193.0, 148.0, 132.0, 131.0, 123.0, 119.0, 28.5, 17.1 ppm.
3.3. Reaction of 12 with N-methyltriphenyl- phosphonylidenesuccimide (6a) in acetic acid
- A mixture of dialdehyde 12 (212 mg, 1.00 mmol) and phosphorane reagent 6a (373 mg, 1.00 mmol) in 5 mL of AcOH was refluxed on an oil bath for 19 h under nitrogen atmosphere. The resulted reaction mixture was poured into cold water (100 mL) and was extracted with chloroform (20 mL x 3). The combined organic layer was washed with a saturated NaHCO3 solution and brine, and was dried over Na2SO4. The solvent was removed under reduced pressure and the residue was purified by silica gel chromatography (AcOEt/CHCl3 =3/97) to give 179 mg (60%) of 15 and 36 mg (9%) of 16.15: Orange microcrystals, m.p. 210–212°C. IR (ATR) νmax = 1760 (m), 1691 (vs), 1658 (s) cm–1; 1H NMR (CDCl3, 500 MHz) δ = 9.32 (s, 1H), 7.15 (t, J = 2.0 Hz, 1H), 7.11 (dd, J = 10.3, 6.1 Hz, 1H), 7.10 (s, 1H), 7.06 (dd, J = 10.3, 6.1 Hz, 1H), 6.87 (d, J = 6.0 Hz, 1H), 6.83 (s, 1H), 6.71 (d, J = 6.1 Hz, 1H), 4.43 (dd, J = 21.8, 2.0 Hz, 1H), 4.41 (d, J = 15.8 Hz, 1H), 4.07 (d, J = 12.2 Hz, 1H), 3.80 (d, J = 15.8 Hz, 1H), 3.62 (dd, J = 21.8, 2.0 Hz, 1H), 3.09 (s, 3H), 0.49 (d, J = 12.2 Hz, 1H) ppm; 13C NMR (CDCl3, 125 MHz) δ= 194.2, 174.6, 171.7, 148.4, 139.5, 139.2, 131.9, 131.0, 130.0, 129.5, 123.5, 121.4, 120.9, 120.0, 117.6, 34.2, 28.2, 24.9, 22.1 ppm; UV-vis (CH3OH) λmax = 249 (logε = 4.05), 312 (4.74), 397 (3.86) nm; MS (70 eV) m/z (rel int) 307 (M+, 100), 278 (29), 204 (15), 194 (20), 193 (68), 191 (23), 189 (15), 179 (36), 178 (57), 169 (32), 165 (37), 153 (18), 152 (24), 141 (20), 128 (15), 115 (16), 89 (15). HRMS Calcd for C19H17NO3, 307.1208, found 307.1208.16: Orange microcrystals, m.p. 298–300°C. IR (ATR) νmax = 1758 (s), 1689 (vs) cm–1; 1H NMR (CDCl3, 400 MHz) δ = 7.12 (t, J = 2.0 Hz, 2H), 7.03 (m, 2H), 6.75 (s, 2H), 6.70 (m, 2H), 4.48 (d, J = 16.0 Hz, 1H), 3.91 (d, J = 12.5 Hz, 1H), 3.54 (dd, J = 21.5, 2.0 Hz, 2H), 3.45 (d, J = 16.0 Hz, 1H), 3.43 (dd, J = 21.5, 2.0 Hz, 1H), 3.09 (s, 6H), 0.36 (d, J = 12.5 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz) δ = 173.8, 170.9, 138.8, 135.1, 130.2, 129.4, 123.1, 118.9, 116.5, 34.0, 33.5, 27.8, 24.9 ppm; UV-vis (CH3OH) λmax = 333 (logε = 4.14), 407 (3.42) nm; MS (70 eV) m/z (rel int) 402 (M+, 20), 290 (14), 265 (20), 264 (100), 215 (22), 205 (20), 203 (16), 202 (20), 193 (15), 191 (18), 189 (18), 179 (23), 178 (23), 165 (31), 152 (17), 58 (15). HRMS Calcd for C24H22N2O4, 402.1580, found 402.1580.
3.4. Synthesis of 10a from 15
- To a solution of sodium methoxide (0.075 mmol) in 3 mL of methanol, prepared with 3.6 mg of 50% sodium hydride and methanol, was added 77.0 mg (0.25 mmol) of 15 in one portion. This mixture was refluxed on an oil bath for 5 h under nitrogen atmosphere. The resulted reaction mixture was poured into 0.1M HCl (40 mL) and was extracted with chloroform (15 mL x 3). The combined organic layer was washed with a saturated NaHCO3 solution and brine, and was dried over Na2SO4. The solvent was removed under reduced pressure and the residue was purified by silica gel chromatography (AcOEt/CHCl3 =1/99) to give 62 mg (86%) of 10a as orange microcrystals. M.p. 234–236°C. IR (ATR) νmax = 1752 (m), 1692 (s) cm–1; 1H NMR (CDCl3, 400 MHz) δ = 8.41 (s, 2H), 8.04 (s, 2H), 7.74 (m, 2H), 7.49 (m, 2H), 3.20 (s, 3H), 1.34 (d, J = 14.0 Hz, 1H), 1.00 (d, J = 13.5 Hz, 1H), –0.32 (d, J = 14.0 Hz, 1H), –1.27 (d, 13.5 Hz, 1H) ppm; UV-vis (CH3OH) λmax = 238 (logε = 4.05), 278sh (4.15), 300sh (4.41), 334 (4.90), 396 (4.04), 465sh (2.72), 493 (2.77), 530 (2.89) nm; MS (70 eV) m/z (rel int) 289 (M+, 100), 274 (18), 204 (39), 203 (36), 202 (32), 189 (17). HRMS Calcd for C19H15NO2, 289.1103, found 289.1099.
3.5. One-pot synthesis of 10a from 12
- A mixture of 12 (21 mg, 0.10 mmol), 6a (56 mg, 0.15 mmol) and sodium methoxide (8 mg, 0.15 mmol) in methanol (3 mL) was refluxed on an oil bath for 8 h under nitrogen atmosphere. The resulted reaction mixture was poured into 0.1M HCl (20 mL) and was extracted with chloroform (10 mL x 3). The combined organic layer was washed with a saturated NaHCO3 solution and brine, and was dried over Na2SO4. The solvent was removed under reduced pressure and the residue was purified by silica gel chromatography (AcOEt/CHCl3 =1/99) to give 12 mg (42%) of 10a as orange microcrystals.
3.6. One-pot Synthesis of 10b from 12
- A mixture of 12 (21 mg, 0.10 mmol), 6b (54 mg, 0.15 mmol) and sodium methoxide (11 mg, 0.20 mmol) in methanol (3 mL) was refluxed on an oil bath for 8 h under nitrogen atmosphere. The resulted reaction mixture was poured into 0.1M HCl (30 mL) and was extracted with chloroform (10 mL x 3). The combined organic layer was washed with a saturated NaHCO3 solution and brine, and was dried over Na2SO4. The solvent was removed under reduced pressure and the residue was purified by silica gel chromatography (AcOEt/CHCl3 =1/9) to give 14 mg (52%) of 10b as orange solids. M.p. 186–188°C. IR (ATR) νmax = 1764 (s), 1752 (s), 1692 (s) cm–1; 1H NMR (CDCl3, 400 MHz) δ = 8.44 (s, 2H), 8.09 (s, 2H), 7.90 (br, 1H), 7.77 (m, 2H), 7.50 (m, 2H), 1.95 (d, J = 14.1 Hz, 1H), 0.97 (d, J =13.2 Hz, 1H), –0.44 (d, J = 14.1 Hz, 1H), –1.22 (d, J = 13.2 Hz, 1H) ppm; 13C NMR (CDCl3, 100 MHz) δ = 170.1, 138.6, 137.7, 135.6, 129.6, 128.6, 107.1, 107.0, 26.3, 25.0 ppm; UV-vis (CH3CN) λmax = 272 (logε = 3.88), 300sh (4.31), 328 (4.85), 330 (4.86), 393 (3.94), 495 (2.67), 527 (2.80), 533 (2.79) nm; MS (70 eV) m/z (rel int) 275 (M+, 100), 260 (17), 204 (25), 203 (28), 202 (26), 189 (14), 101 (17). HRMS Calcd for C18H13NO2, 275.0946, found 275.0947.
3.7. X-ray Diffraction Analysis of 10a
- Diffraction measurements were conducted using a Rigaku R-AXIS RAPID diffractometer at –100°C. Crystal data for 10a are as follows; monoclinic, space group; P21/n (# 14), a; 9.32224(17) Å, b; 12.2859(3) Å, c; 24.6428(5) Å, β; 90.3387(7)°, V; 2822.35(9) Å3, Z; 8, R; 0.0488, wR2; 0.1225, R1; 0.0448 (I>2.00σ(I)), and S; 1.038. Tables of fractional atomic coordinates, thermal parameters, bond lengths, and angles have been deposited at the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, United Kingdom (CCDC 1048825) [Direct line: +44 1223 762910, Fax: +44 (0) 1233 336033, e-mail: deposit@ccdc.cam.ac.uk].
4. Conclusions
- We have demonstrated an alternative shortcut synthesis of dialdehyde 12 from 5 by double aldol condensation with glutaraldehyde and also accomplished synthesis of the title compounds 10a-b from 12. It should be stressed that a dimethano[14]annulene derivative can be synthesized in two steps from 5. The solid-state structure of 10a was determined by X-ray diffraction analysis, revealing that annulation of the dicarboximide moiety on a dimethano- [14]annulene core dose not affect bond-convergency of the annulene ring. Upon photoexcitation 10a-b show emission but their quantum yields are less than those of 1 and 2.
ACKNOWLEDGEMENTS
- We thank Ms Yurie Fujiwara at Shinshu University and Mr Isao Hirano at University of Toyama for their technical assistance. A financial support from the Faculty of Science in Shinshu University (for M.O.) is greatly acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML