-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2015; 5(3): 95-104
doi:10.5923/j.ajoc.20150503.02
Synthesis and Characterization of New 1, 2, 4- (Triazine) Thio Benzoxazole Derivatives
Mohammad R. Ahmed, Ali A. Mohsin
Department of Chemistry, College of science, Baghdad University, Baghdad, Iraq
Correspondence to: Ali A. Mohsin, Department of Chemistry, College of science, Baghdad University, Baghdad, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This research, involved a series of some new compounds containing different heterocyclic atoms, through synthesis of 2-mercaptobenzoxazole (2-MBO) (A) by using homemade Auto clave. The synthesis involved treatment of 2-MBO with 2-Chloroacetyl chloride to give 2-Chloroacetyl thio benzoxazole (A1), the product was treated with hydrazine hydrate to give 2-(aceto hydrazide) thio benzoxazole (A2). Synthesis of 2-(aceto phenyl semi carbazide) thio benzoxazole (A3) yielded from reaction of 2-(aceto hydrazide) thio benzoxazole (A2) with phenyl isocyanate, and then compound (A3) was converted to the corresponding cyclic semicarbazide (A4) in presence of 30% of sodium hydroxide. After that, 2- (4-phenyl-1,2,4-triazine-3-one) thio benzoxazole (A4) introduced in the reaction with different alkyl halides and benzoyl chloride to synthesized the corresponding (A5-A12)compounds respectively. Structure of all the prepared compounds confirmation were proved using (FT-IR), (1H-NMR) and (13C-NMR) spectra in addition to melting points.
Keywords: Benzoxazole, 1,2,4-Triazine, Heterocyclic synthesis
Cite this paper: Mohammad R. Ahmed, Ali A. Mohsin, Synthesis and Characterization of New 1, 2, 4- (Triazine) Thio Benzoxazole Derivatives, American Journal of Organic Chemistry, Vol. 5 No. 3, 2015, pp. 95-104. doi: 10.5923/j.ajoc.20150503.02.
Article Outline
1. Introduction
- Benzoxazole is an important heterocyclic ring system and the targets containing benzoxazole moiety, either isolated from natural products or accessed by total synthesis, possess most remarkable and a wide range of biological activity [1]. The benzoxazole contains a phenyl ring fused to an oxazole ring, this important moiety has found practical application in a number of fields [2]. Benzoxazole nucleus reported various types of biological activity such as anti-bacterial [3], anti-inflammatory [4], anti-cancer [5], anti-microbial [6], anti-fungal [7], anti-biotic [8]. 2-mercaptobenzoxazole are the thiol derivatives of simple nucleus, they exist in two tautomeric forms i.e. thiol and thion [9].
2. Experimental
2.1. Chemicals
- All the chemicals used with high purity as the manufactures supplied starting chemical compounds were obtained from BDH, Sigma Aldrich, Fluka and used as received.
2.2. Instruments
- Instruments were used: 1H-NMR and 13C-NMR spectrum were recorded on Burker 300 MHz instrument using DMSO-d5 as solvent and TMS as internal reference, The FT-IR spectrum in the range (4000-200) cm-1 were recorded as KBr disc on a Shimadzu FT-IR 8300 spectrophotometer. The 2- MBO was prepared by using the manufacturer domestic autoclave made from stainless steel with a capacity of 300 ml and of 12.5 cm diameter as shown below in Figure (1).
 | Figure (1). The manufacturer domestic Autoclave |
2.3. General Procedures
2.3.1. Synthesis of 2-Mercaptobenzoxazole(2-MBO) (A)
- 2-Amino phenol (10.91g, 0.1mol.) was mixed with (100 ml) absolute ethanol and (6.19ml, 0.1mol.) Carbon disulfide. The mixture was transferred into Autoclave and then closing it very well to ensure getting a high temperature and pressure. This set-up was heated in a sand bath at 180°C for (6-8) hr., then the resulted mixture was placed in a beaker containing (7ml) of 10% Sodium hydroxide to remove unreacted amine. Few drops of concentrated hydrochloric acid was added until the mixture became acidic to precipitate thiol. The precipitate was filtered off and followed by addition of (7 ml) 25% sodium carbonate dried and recrystallized from ethanol. The physical properties of compound (A) are listed in Table (1).
2.3.2. Synthesis of 2-Chloroacetyl Thio Benzoxazole (A1)
- Equimolar of 2-Mercaptobenzoxazole(A) (15.11g, 0.1 mol.) with chloroacetyl chloride (7.96 ml, 0.1 mol.) in dry chloroform (50 ml) in the presence of trace quantities anhydrous potassium carbonate were refluxed on a water bath for about (14) hr. The solvent was removed by vacuum. The residue was recrystallized from methanol. The physical properties of compound (A1) are listed in Table (1).
2.3.3. Synthesis of 2-(Aceto Hydrazide) Thio Benzoxaz- ole (A2)
- A mixture of 2-Chloroacetyl thio benzoxazole (A1) (22.76g, 0.1mol) and hydrazine hydrate (4.9 ml, 0.1mol) in (50ml) absolute ethanol were stirred at room temperature for about (5) hr. The solvent was removed under reduced pressure and the residue to offer the product. The solid precipitate was filtered off and recrystallized from benzene. The physical properties of compound (A1) are listed in Table (1).
2.3.4. Synthesis of 2-(Aceto Phenyl Semi Carbazide) Thio Benzoxazole (A3)
- To asolution of 2-(acetohydrazide)thio benzoxazole (A2) (2.23 g., 0.01mol.) in absolute ethanol (15 ml), phenylisocynate (1 ml, 0.01 mol.) was added with continuous stirring and the mixture was refluxed for (3-4) hr. The reaction mixture was cooled and the formed solid precipitate was filtered off and recrystallized from benzene. The physical properties of compound (A3) are listed in Table (1).
2.3.5. Synthesis of 2- (4-phenyl-1,2,4-triazine-3-one) Thio Benzoxazole (A4)
- 2-(aceto phenyl semicarbazide) thio benzoxazole (A3) (3.42gm., 0.01mol.) was refluxed with 30% aqueous sodium hydroxide solution (100ml) for (3-4) hr. The reaction mixture was filtered, cooled, and neutralized by gradual addition with stirring of 10% acetic acid solution. The formed precipitate was filtered and recrystallized from ethanol. The physical properties of compound (A4) are listed in Table (1).
2.3.6. Synthesis of 2- (4-phenyl-2-N-substituted 1,2,4-triazine-3-one) thio benzoxazole (A5-A10) and 2- (4-phenyl-1,2,4-triazine-3-substituted) Thio Benzoxazole (A11,A12).
- To a stirred solution at (50°C) of 2- (4-phenyl-1, 2,4-triazine-3-one)thio benzoxazole (A4) (3.24gm., 0.01 mol.) in absolute ethanol (20 ml) and KOH (0.56 gm.) was added during 20 min. with stirring, then (1.15ml, 0.01 mol.) of benzoyl chloride or quantitative different alkyl halides was added drop wise and the reaction mixture was refluxed for (3-4) hr. The mixture was filtered, cooled, and poured into cold water then the resulting aqueous layer was extracted with chloroform. The combined chloroform extracts was evaporated to give the desired product that recrystallized from dry benzene. The physical properties of compounds (A5-A12) are listed in Table (1).
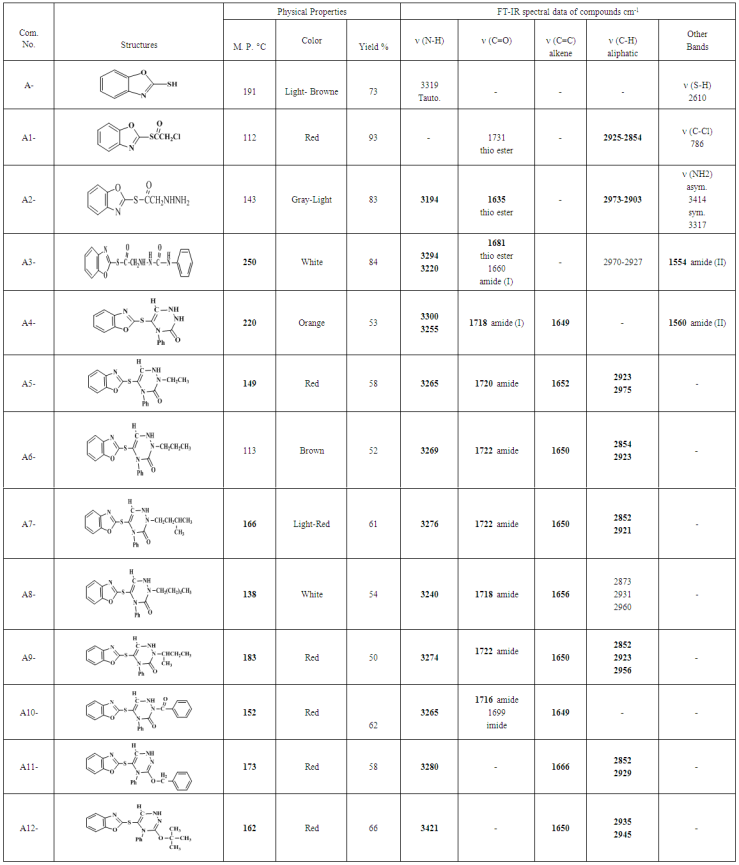 | Table (1). Physical Properties and FT-IR Spectral Data of the Prepared Compounds (A-A12) |
3. Result and Discussion
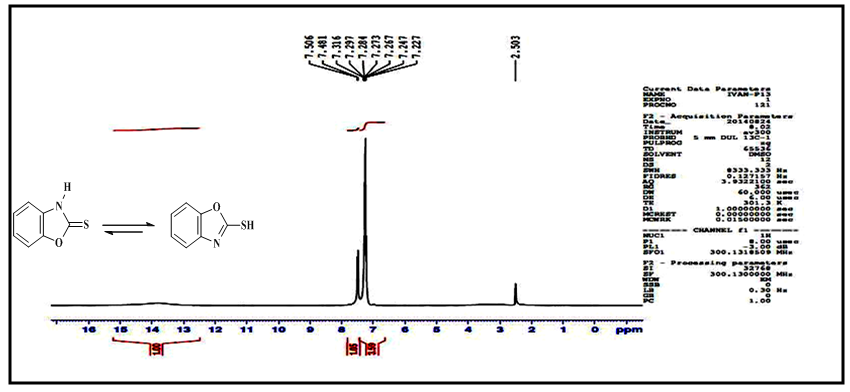
- 2-MBO was obtained from the reaction of 2-aminophenol with carbon disulfide in absolute ethanol by using closed system. This method was selected, because it gave 2-MBO in a good yield and high purity [10]. The FT-IR spectrum showed clear absorption bands at ν(3319) cm-1 due to ν (N-H), (3068,3039)cm-1 due to ν(C-H) aromatic, (1618) cm-1 due toν(C=N) oxazole ring, (1506,1446) cm-1 due to ν(C=C) aromatic and (2610) cm-1 due to ν(S-H) and disappearance of the two absorption band in the range of (3376, 3305) cm-1 which could be attributed to asymmetric and symmetric stretching vibration (NH2) group of 2-aminophenol [11, 12]. All details of FT-IR spectral data of compound (A) are listed in Table (1). 1H-NMR spectrum of compound (A) showed singlet signals at δ= (13.80) due to
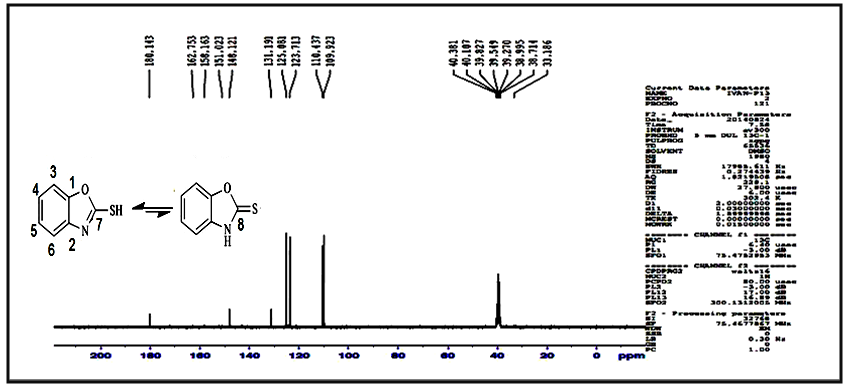
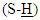 proton and multi signal at δ= (7.22-7.50) ppm due to aromatic protons. 1H-NMR spectral data of compound (A) are shown in Figure (2). 13C-NMR spectrum of compound (A) showed signals at δ= (109.92-131.19) ppm, δ= (148.12) ppm, and δ= (180.14) ppm, belong to (C=C) aromatic carbon,
proton and multi signal at δ= (7.22-7.50) ppm due to aromatic protons. 1H-NMR spectral data of compound (A) are shown in Figure (2). 13C-NMR spectrum of compound (A) showed signals at δ= (109.92-131.19) ppm, δ= (148.12) ppm, and δ= (180.14) ppm, belong to (C=C) aromatic carbon, 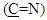 and
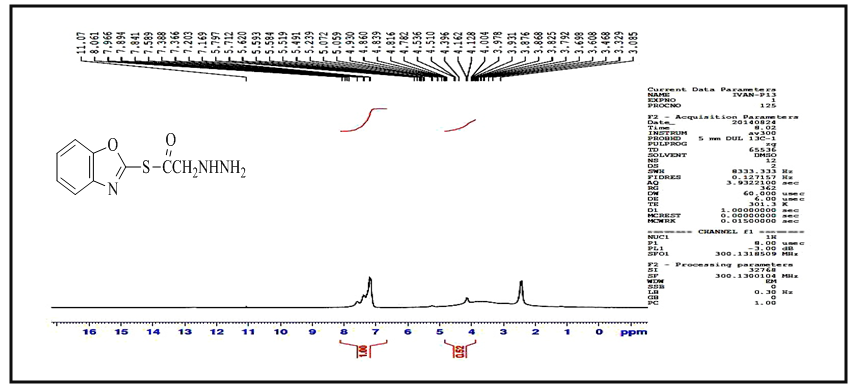
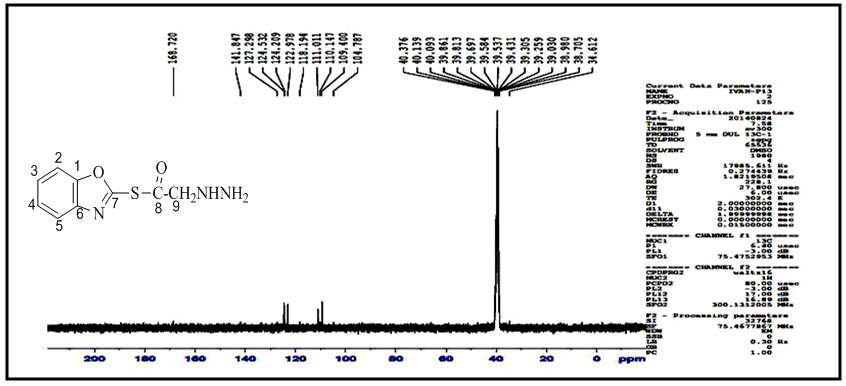
and 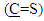 respectively. 13C-NMR spectral data of compound (A) are shown in Figure (3).The reaction 2-mercaptobenzoxazole with 2-Chloroacetyl chloride in alkali media (K2CO3) was used to prepare the compound (A1). The FT-IR spectrum of compound (A1) showed the appearance of absorption bands at (2925, 2854) cm-1 due to ν(C-H) aliphatic, absorption band at (1731) cm-1 due to ν(C=O) while absorption band due to ν(C-Cl) at (786) cm-1, these bands and others are shown in Table (1), then reaction 2-Chloro acetyl thio benzoxazole (A1) was stirred with hydrazine hydrate to give 2-( aceto hydrazide) thio benzoxazole (A2). The FT-IR spectrum of compound (A2) showed appearance of characteristic absorption bands at (3414, 3317) cm-1 belong to ν(NH2) asym. and sym., characteristic absorption band at (1635) cm-1 belong to ν(C=O) and disappearance of the absorption band at (786) cm-1 belong to ν (C-Cl), as shown in Table (1). 1H-NMR spectrum data of compound (A2) showed broad hump at δ= (3.08-4.00) ppm due to the three protons of the hydrazine moiety, multi signals at δ= (3.32) ppm due to
respectively. 13C-NMR spectral data of compound (A) are shown in Figure (3).The reaction 2-mercaptobenzoxazole with 2-Chloroacetyl chloride in alkali media (K2CO3) was used to prepare the compound (A1). The FT-IR spectrum of compound (A1) showed the appearance of absorption bands at (2925, 2854) cm-1 due to ν(C-H) aliphatic, absorption band at (1731) cm-1 due to ν(C=O) while absorption band due to ν(C-Cl) at (786) cm-1, these bands and others are shown in Table (1), then reaction 2-Chloro acetyl thio benzoxazole (A1) was stirred with hydrazine hydrate to give 2-( aceto hydrazide) thio benzoxazole (A2). The FT-IR spectrum of compound (A2) showed appearance of characteristic absorption bands at (3414, 3317) cm-1 belong to ν(NH2) asym. and sym., characteristic absorption band at (1635) cm-1 belong to ν(C=O) and disappearance of the absorption band at (786) cm-1 belong to ν (C-Cl), as shown in Table (1). 1H-NMR spectrum data of compound (A2) showed broad hump at δ= (3.08-4.00) ppm due to the three protons of the hydrazine moiety, multi signals at δ= (3.32) ppm due to 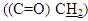 protons and multi signals at δ= (7.16-8.06) ppm due to aromatic protons. 1H-NMR Spectral data of compound (A2) are shown in Figure (4). 13C-NMR spectrum data of compounds (3) showed signals at δ = (34.61) ppm due to
protons and multi signals at δ= (7.16-8.06) ppm due to aromatic protons. 1H-NMR Spectral data of compound (A2) are shown in Figure (4). 13C-NMR spectrum data of compounds (3) showed signals at δ = (34.61) ppm due to 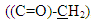 , δ = (141.84) ppm due to
, δ = (141.84) ppm due to 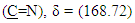 ppm due to
ppm due to 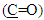 and signals δ= (104.78-127.29) ppm due to (C=C) aromatic carbon.13C-NMR Spectral data of compound (A2) are shown in Figure (5).
and signals δ= (104.78-127.29) ppm due to (C=C) aromatic carbon.13C-NMR Spectral data of compound (A2) are shown in Figure (5).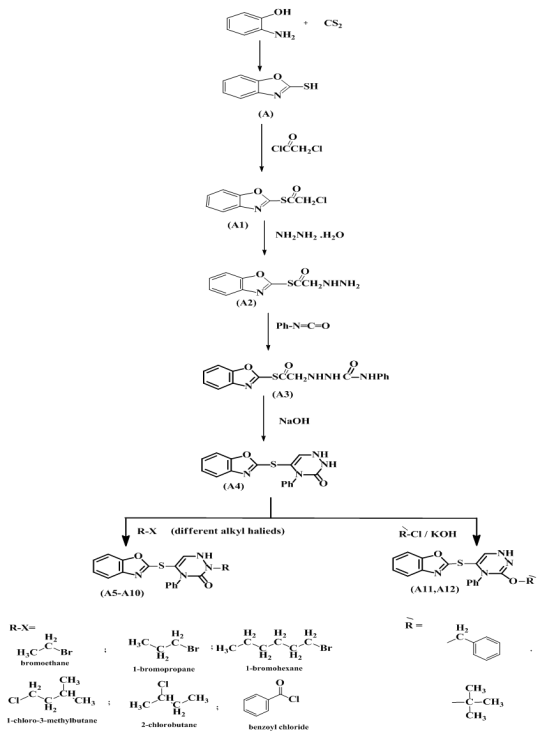 | Scheme 1. The scheme of prepared compounds |
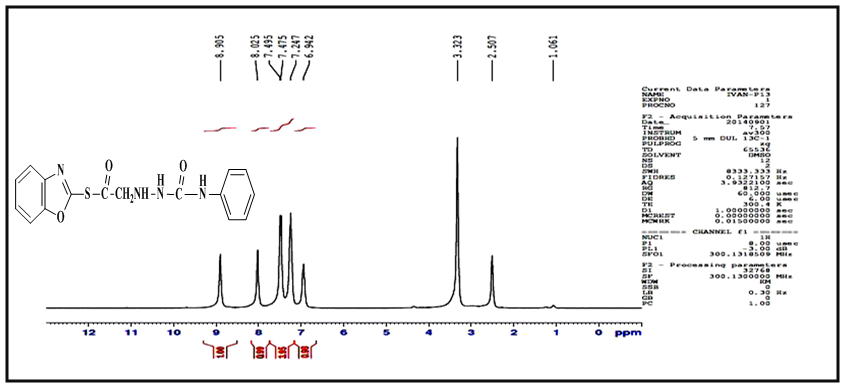
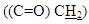 protons, singlet signal at δ= (6.94) ppm due to
protons, singlet signal at δ= (6.94) ppm due to 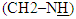 proton, two singlet signal at δ= (8.02, 8.90) ppm due to
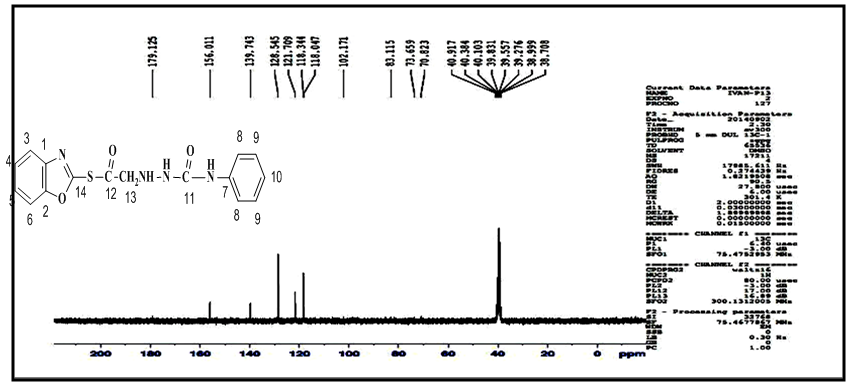
proton, two singlet signal at δ= (8.02, 8.90) ppm due to 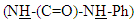 protons, and signals at δ= (7.24-7.49) ppm due to aromatic protons. 1H-NMR spectrum of compound (A3) are listed in Table (2) and showed in Figure (6). 13C-NMR spectrum data of compound (4) showed signals at δ= (40.91) ppm, δ= (83.11-128.54) ppm, δ= (156.01) and at δ = (179.12) ppm belong to
protons, and signals at δ= (7.24-7.49) ppm due to aromatic protons. 1H-NMR spectrum of compound (A3) are listed in Table (2) and showed in Figure (6). 13C-NMR spectrum data of compound (4) showed signals at δ= (40.91) ppm, δ= (83.11-128.54) ppm, δ= (156.01) and at δ = (179.12) ppm belong to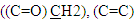 aromatic carbon,
aromatic carbon, 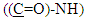 and
and 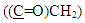 respectively, 13C-NMR spectrum of compound (A3) are listed in Table (3) and showed in Figure (7). Treatment of compound (A3) with (30%, NaOH) solution affords intramolecular cyclization to give 2-(4-phenyl-1,2,4-triazine-3-one) thio benzoxazole (A4), Mechanism [13] of reaction involved nucleophilic substitutions lead to intramolecular cyclization by SN2 mechanism as shown in Scheme (2). The FT-IR spectrum of compound (A4) showed disappearance of absorptions bands due to ν(CH2) aliphatic absorption bands at (2927, 2970) cm-1 and ν(C=O thio ester) at (1681) cm-1, the spectrum showed starching bands at (3300) cm-1, (1649) cm-1 and (1718) cm-1 due to ν(N-H), ν(C=C) and ν(C=O, amide(I)) respectively. All details of FT-IR Spectral data of compound (A4) are listed in Table (1).
respectively, 13C-NMR spectrum of compound (A3) are listed in Table (3) and showed in Figure (7). Treatment of compound (A3) with (30%, NaOH) solution affords intramolecular cyclization to give 2-(4-phenyl-1,2,4-triazine-3-one) thio benzoxazole (A4), Mechanism [13] of reaction involved nucleophilic substitutions lead to intramolecular cyclization by SN2 mechanism as shown in Scheme (2). The FT-IR spectrum of compound (A4) showed disappearance of absorptions bands due to ν(CH2) aliphatic absorption bands at (2927, 2970) cm-1 and ν(C=O thio ester) at (1681) cm-1, the spectrum showed starching bands at (3300) cm-1, (1649) cm-1 and (1718) cm-1 due to ν(N-H), ν(C=C) and ν(C=O, amide(I)) respectively. All details of FT-IR Spectral data of compound (A4) are listed in Table (1).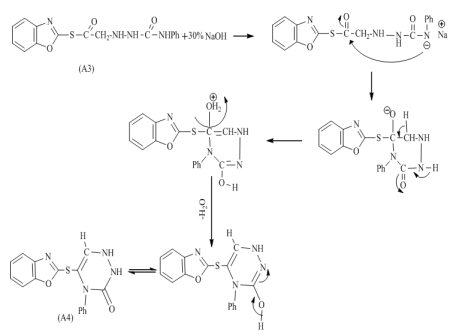 | Scheme 2. Mechanism of prepared compound (A4) |
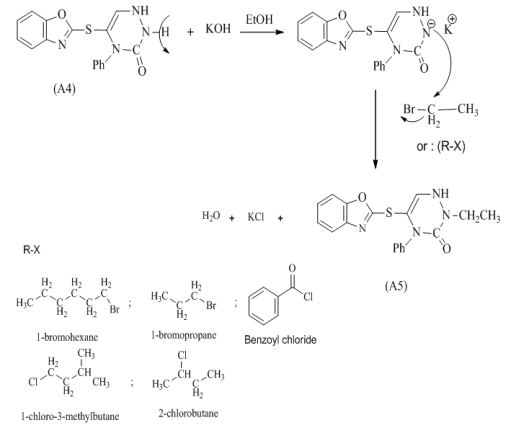 | Scheme 3. Mechanism of prepared compound (A5-A10) |
 | Figure (2). 1H-NMR spectrum of compound (A) |
 | Figure (3). 13C-NMR spectrum of compound (A) |
 | Figure (4). 1H-NMR spectrum of compound (A2) |
 | Figure (5). 13C-NMR spectrum of compound (A2) |
 | Figure (6). 1H-NMR spectrum of compound (A3) |
 | Figure (7). 13C-NMR spectrum of compound (A3) |
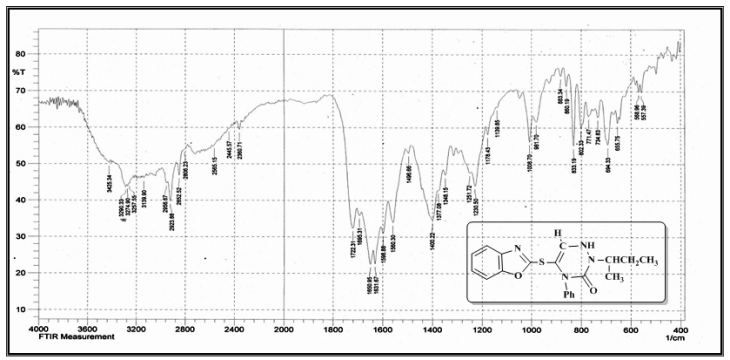 | Figure (8). FT-IR spectrum of compound (A9) |
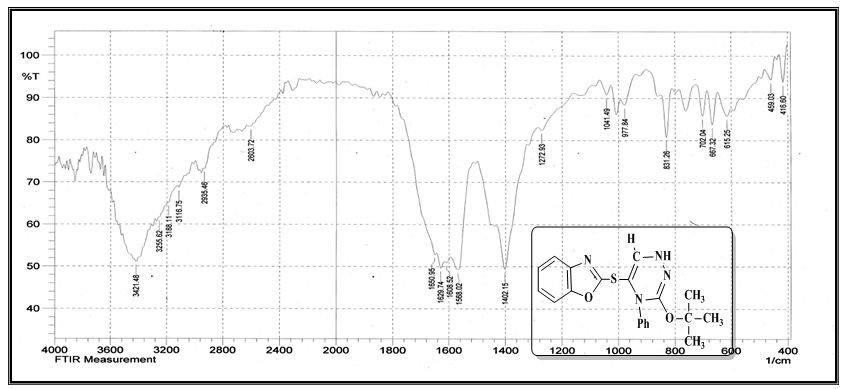 | Figure (9). FT-IR spectrum of compound (A12) |
4. Conclusions
- This research included new methodology for synthesis of 2-mercaptobenzoxazole, by using closed system (Autoclave). Also, new derivatives of 1,2,4 triazine ring were synthesized. All these compounds were characterized by different spectral studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML