-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2015; 5(2): 78-81
doi:10.5923/j.ajoc.20150502.03
Tandem Catalysis of New Aza Angular Phenothiazine Ring Systems
E. L. Ayuk1, A. N. Njokunwogbu1, S. U. Ilo1, G. A. Engwa1, U. C. Okoro2, T. O. Oni3
1Department of Chemical Science, Faculty of Natural and Applied Sciences Godfrey Okoye University, Thinkers Corner, Enugu, Nigeria
2Department of pure and Industrial chemistry, Faculty of Physical Sciences University of Nigeria, Nsukka, Nigeria
3School of General studies, Delta State Polytechnic Ogwashi – Uku, Delta State, Nigeria
Correspondence to: E. L. Ayuk, Department of Chemical Science, Faculty of Natural and Applied Sciences Godfrey Okoye University, Thinkers Corner, Enugu, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This communication deals with tandem synthesis of new aza angular phenothiazine ring systems of industrial importance via Suzuki-Miyaura reaction. 1,4-Bis-(2-hydroxy-3,5-di-tert-butyl)piperazine (ligand) was obtained by treating 40% paraformaldehyde solution, piperazine and 2,4-di-tert-butylphenol. This was combined with diphenylphospinobutane chloride Pd(dppb)Cl to form a complex catalyst system. 2-Aminopyridine-3-thiol one of the key intermediates was obtained through thiocyanation of 2-aminopyridine followed by alkaline hydrolysis of 2-amino-3-thiocyanatopyridine. 2-Aminopyridine-3-thiol was then treated with 2,3-dichloro-1,4-naphthoquinone to give a new aza angular phenothiazine ring which coupled with 3-chlorophenyl boronic acid in the presence of the complex catalyst system above to furnish another new phenothiazine polycyclic ring system 6-(3-chlorophenyl)-11-azabenzo[a] phenothiazin-5-one.
Keywords: Suzuki-Miyaura reaction, Ligand, 2-aminopyridine-3-thiol, Thiocyanation
Cite this paper: E. L. Ayuk, A. N. Njokunwogbu, S. U. Ilo, G. A. Engwa, U. C. Okoro, T. O. Oni, Tandem Catalysis of New Aza Angular Phenothiazine Ring Systems, American Journal of Organic Chemistry, Vol. 5 No. 2, 2015, pp. 78-81. doi: 10.5923/j.ajoc.20150502.03.
Article Outline
1. Introduction
- Phenothiazine and its derivatives make up an important class of bioactive heterocyclic compounds, which have wide spectrum of industrial biological and pharmacological potentials [1-6]. They have been found to possess promising medical activities as tranquilizers, analgesics, sedatives, antimalarial, anticancer, antibacterial, antifungal, antidepressants, antipsychotics etc. They have also been found useful in paints, textile, agricultural, petroleum industries as dyes and pigments, pesticides antioxidants respectively [7-8]. Any variation in the substitution pattern of the phenothiazine ring brings about a difference in both its biological and industrial applicability [9]. There is a global search for such heterocyclic ring systems which have wide applications as mention above, to solve numerous health and industrial problems affecting mankind. As a way forward in contributing to the above search, we have successfully synthesis 6-chloro-11-azabenzo[a]phenothiazin-5-one 1 and 6-(3-chlorophenyl)-11-azabenzo[a]pheonthiazin-5-one 2.
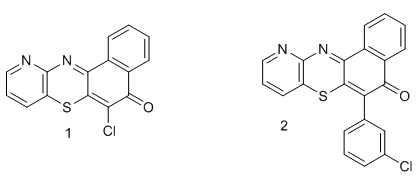 Similar compounds like the type 1 were reported by W.B Kang and co-workers [10-11]. However, they observed the formation of mixture of products of the type 5 and 6 when aminobenzothiols were reacted with substituted naphthoquinones due the presence of substituents at the sixth or seventh positions of thenaphthoquinones used as shown below;
Similar compounds like the type 1 were reported by W.B Kang and co-workers [10-11]. However, they observed the formation of mixture of products of the type 5 and 6 when aminobenzothiols were reacted with substituted naphthoquinones due the presence of substituents at the sixth or seventh positions of thenaphthoquinones used as shown below;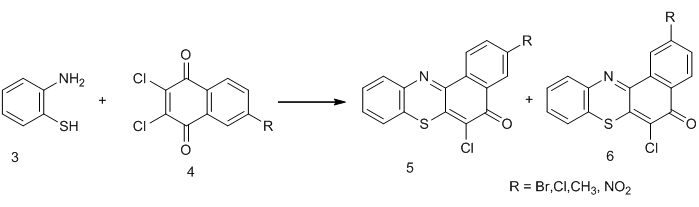 From the above, it could observed that stereo-selective products are obtained by making use of non-substituted naphthoquinones, as was done in the synthesis of compound 1 above.
From the above, it could observed that stereo-selective products are obtained by making use of non-substituted naphthoquinones, as was done in the synthesis of compound 1 above.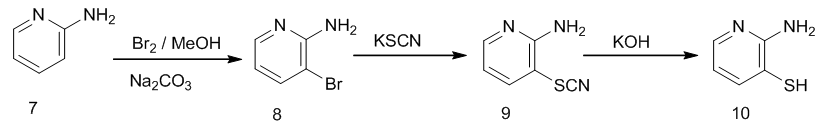 | Scheme 1. Synthesis of 2-aminopyridine-3-thiol 10 |
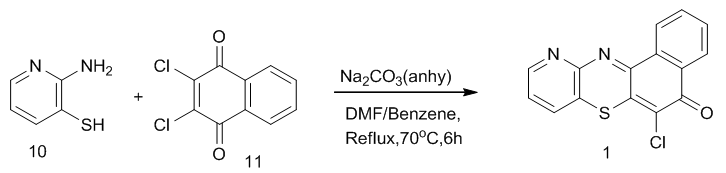 | Scheme 2. Synthesis of 6-chloro-11-azabenzo[a]phenothiazin-5-one 1 |
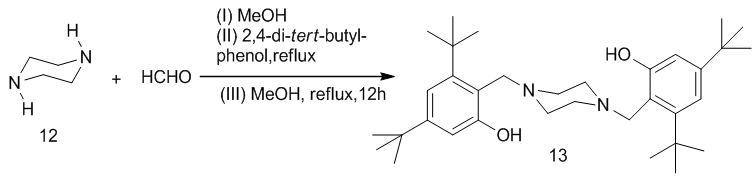 | Scheme 3. Synthesis of 1,4-Bis-(2-hydroxy-3,5-di-tert-butylbenzyl) piperazine 13 |
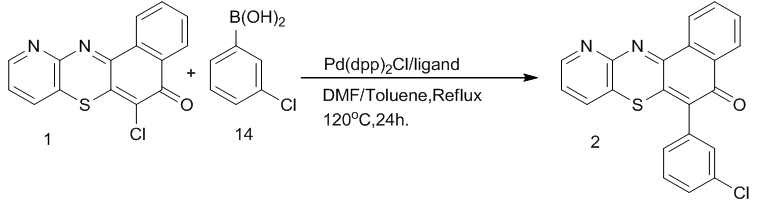 | Scheme 4. Synthesis of 6-(3-chlorophenyl)-11-azabenzo[a]phenothiazin-5-one 2 |
2. Materials and Methods
- All the melting points of the synthesized compounds were determined in open capillary tubes and are uncorrected. IR spectra were recorded in KBr on a Fourier transform infrared spectrophotometer.UV-Vis spectra in DMF on a Jenway 640 UV-Vis spectrophotometer using matched 1cm quartz cells. 1H-NMR and 13C-NMR were recorded on JEOL Al-400 spectrophotometer (400 MHz) in CDCl3/DMSO-d6 using TMS as an internal standard (chemical shifts are measured in δ ppm). Mass spectra were recorded on a Shimadzu QP2010 spectrophotometer. The purity of the synthesized compounds were checked by column chromatography using aluminum oxide 90 (Merked, 70-230 Mesh ASTM) with mixture of benzene and chloroform (1:1) as eluents before recrystallization. Compound 7-10 and 13 were prepared as described in literature [12-13] and [14] respectively.6-Chloro-11-azabenzo[a]phenothiazin-5-one (1)A mixture of 2-aminopyridine-3-thiol 10(2.0g, 0.0159mol) and anhydrous sodium carbonate (2.6g, 0.0245mol) was placed in a reaction flask equipped with a magnetic stirrer, thermometer and a reflux condenser. A solution of benzene (50ml) and DMF (5ml) was added and the mixture refluxed for 1h for dissolution. 2, 3-Dichloro-1, 4-naphthoquinone11 (2.6g, 0.0115mol) was later added and the entire mixture refluxed with continuous stirring for 7h at 70-75oC. At the end of the refluxing period benzene was distilled off and the slurry poured into water and stirred to dissolve the inorganic materials. It was cooled, filtered, dried and subjected to column chromatography on aluminum oxide using benzene and chloroform (1:1) as eluent. The first yellow band eluted was the unreacted 1,4-naphthoquinone. The second reddish band was collected and recrystallized from acetone after treatment with activated charcoal to yield compound (1) as a reddish crystalline product, m.p>190oC (3.46g 71%). UV/Visλmax: 327(ε=1.844)nm, 334(ε=1.934)nm. IR (KBr) 1672cm-1 (C-O), 3077cm1 (C–H Aromatic), 1559cm-1 (C=N, pyridine), 12636cm-1, 1124cm-1, 811cm-1 and 622cm-1. 1H–NMR (DMSO-d6) δ 8.27(s,10-H),δ 8.05(8-H and 9-H),7.90(m,1-H,2-H,3-H and 4-H).13C-NMR(DMSO-d6) ppm:176.43(C=O),135.21(1C,s),131.48(1C,s), 127.61(1C,s), 40.27 (6C,m),139.89(5C,m).6-(3-Chlorophenyl)-11-azabenzo[a]phenothiazin-5-one (2)In a 250ml two-necked round bottom flask, diphenylphosphinobutane palladium chloride, Pd(dppb)2Cl (0.005mmol) 1,4-bis-(2-hydroxy-3,5-di-tert-butylbenzyl) piperazine (0.005mmol) and mixture of DMF and toluene (10ml) (2:3) were placed and stirred for 5minutes using a short magnetic bar without heating. Thereafter, compound 1(1.047mmol), 3-chlorophenyl boronic acid 14 (0.75mmol), and potassium carbonate (0.11mmol) were their added and the mixture refluxed for 24h. The course of the reaction was monitored with TLC analysis. At the end of the reaction, the mixture was poured into a glass petri dish to evaporate the solvent completely and the resource was allowed to dry. The dried residue was treated with water (10ml) to dissolve the inorganic materials and then extracted with acetone (10ml) to obtain a reddish brown product which was recrystallized from acetone to obtain compound (2), m.p› 95oC (0.38g, 78.9%).UV/Vis λmax (ε): 321 (1.813)nm, 325(1.833)nm, 330(1.861)nm, IR(KBr), 3071cm-1 (C-H Ar), 1664cm1 (C=O), 1578cm-1 (C=N) 1275cm-1, 1080cm-1, 756cm-1. MS:m/z (relative intensity) 375(M+ 100%), 347(M+-CO) 18%, 343 (M+–S)22%, 264 (M+-C6H4Cl)27%, 340(M-Cl) 12%. H-NMR DMSO-d6:δ 8.25(s,10-H),δ 8.10(d,8-H and 9-H),δ7.99(m 4Hs Ar),δ 7.90(s,1-H ortho subst. of the phenyl ring) δ 7.70(s,3Hs of the phenyl ring).
3. Results and Discussion
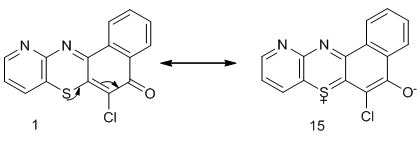
- 2-Aminopyridine 7 was treated with bromine in methanol solution in the presence of sodium carbonate to give 2-amino-3-bromopyridine 8. Compound 8 was further converted to 2-amino-3-thiocyanatopyridine9 with a solution of potassium thiocyanate. Refluxing compound 9 in 20% sodium hydroxide for 30h followed by neutralization with acetic acid gave 2-aminopyridine-3-thiol 10, one of the key intermediates in good yield. This method was described by Okoro and Ofoefule [13].2-Aminopyridine-3-thiol 10 was treated with 2,3-dichloro-1,4-naphthoquinone 11 in the presence of anhydrous sodium carbonate in a mixture of DMF–benzene (1:10) as solvent for 7h to obtain 6-chloro-11-azabenzo[a] phenothiazin-5-one 1 as a reddish crystalline solid melting at 190–191oC after recrystallization from acetone. Micro analysis of IR, 1H-NMR and 13C-NMR are in agreement with the assigned structure. In the IR spectrum there was a lowering of the carbonyl (C=O) absorption from the expected 1700cm-1 to 1672cm1 due to the contribution of the ionic resonance form 15 to ionic resonance effect which increase the (C=O) bond length in 1 with the attendant decrease in the vibration frequency of absorption [13] as shown below.
 To prepare the ligand, a mixture of piperaizine13, 40% paraformaldehyde solution and 2, 4-di-tert-butylphenol was stirred at 60oC in methanol solution for 12h to produce a whitish powdered product in moderate yield melting at 247-249oC (lit.250oC) [14]. This was used with the catalyst (Pd(ddp)2Cl) to formed the palladium catalyst complex for the catalysis of compound 1 and 3-chlorophenyl boronic acid 14. The chlorine atom at the 6th position of the azabenza[a]phenothiazine is substituted by the 3-chlorophenyl moiety of the boronic acid. This forms a C-C sigma bond between the C-6 of the phenothiazin-5-one and C-1 of the boronic acid. Chlorinated compounds have been found to possess fungi static and bacteriostatic activities compared with compounds containing other substituents [15], thus the choice of the 3-chlorophenylboronic. This reaction is known as Suzuki-Miyaura cross coupling reaction [16]. This coupling gave compound 2 in good yield, melting at 94oC–95oC. The micro analysis, IR and mass spectra are in good agreement with the assigned structure.When compound 1 and 2 were treated with sodium dithionite, they gave color discharge unstable leuco-bases 16 and 17 respectively which reverted in the presence of atmospheric oxygen to dehydro compounds. This property makes them suitable as vat dyes.
To prepare the ligand, a mixture of piperaizine13, 40% paraformaldehyde solution and 2, 4-di-tert-butylphenol was stirred at 60oC in methanol solution for 12h to produce a whitish powdered product in moderate yield melting at 247-249oC (lit.250oC) [14]. This was used with the catalyst (Pd(ddp)2Cl) to formed the palladium catalyst complex for the catalysis of compound 1 and 3-chlorophenyl boronic acid 14. The chlorine atom at the 6th position of the azabenza[a]phenothiazine is substituted by the 3-chlorophenyl moiety of the boronic acid. This forms a C-C sigma bond between the C-6 of the phenothiazin-5-one and C-1 of the boronic acid. Chlorinated compounds have been found to possess fungi static and bacteriostatic activities compared with compounds containing other substituents [15], thus the choice of the 3-chlorophenylboronic. This reaction is known as Suzuki-Miyaura cross coupling reaction [16]. This coupling gave compound 2 in good yield, melting at 94oC–95oC. The micro analysis, IR and mass spectra are in good agreement with the assigned structure.When compound 1 and 2 were treated with sodium dithionite, they gave color discharge unstable leuco-bases 16 and 17 respectively which reverted in the presence of atmospheric oxygen to dehydro compounds. This property makes them suitable as vat dyes.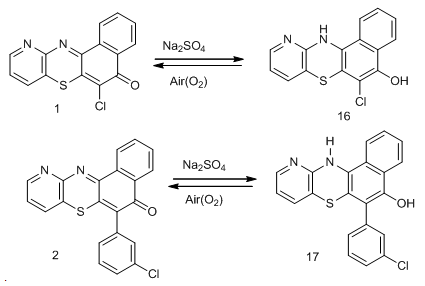
4. Conclusions
- The synthesis of phenothiazine derivatives discussed above was carried out using simple commercially available starting materials. The methods used are straight forward and stereo-selective products were obtained. The catalyst system employed in the synthesis was very effective. These newly synthesis synthesized compounds have promising and interesting applicability in pharmaceutical, textile, petroleum, agricultural industries etc.From the spectroscopic data which are consistent with assigned structures of the above synthesized compounds, their molecular formulae are C15H7ON2SCl and C21H11ON2SCl.
ACKNOWLEDGEMENTS
- The authors wish to thank the following persons, Dr Obasi Nnamdi of University of Strathchyde Laboratories, Glasgow UK, for helping in the running of1H-NMR and 13C-NMR spectra, the staff of NARICT (Zaria) for the IR and mass spectra analysis. The technical assistance of Mr. J.U. Ugwu is also highly appreciated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML