-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2015; 5(2): 57-72
doi:10.5923/j.ajoc.20150502.01
Design, Synthesis of Some New Thio-Substituted Imidazole and Their Biological Activity
Asmaa S. Salman, Anhar Abdel-Aziem, Mrwa J. Alkubbat
Department of Chemistry, Faculty of Science, Al-Azhar University, Girls’ Branch, Cairo, Egypt
Correspondence to: Asmaa S. Salman, Department of Chemistry, Faculty of Science, Al-Azhar University, Girls’ Branch, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In an attempt to find a new class of antimicrobial and antioxidant agents, a two new series of S-alkyl-imidazolidin-4-one(3a,b-5) and S-alkyl-imidazole 13a-d,15a-e,16,17a,b and 25a-d were prepared. Their antioxidant potential were evaluated using DPPH(1,1-diphenyl-2-picrylhydrazyl) radicalmethod in vitro. Their antibacterial screening against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeuroginosa and Escherichia coli and antifungal activity against Aspergillus fumigates, Syncephalastrum racemosum, Geotrichum candidum and Candida albicans were also evaluated, most of the compounds showed to potent and significant results when compared to the respective standards. Structures of the newly synthesized compounds were established by elemental analysis and spectral data.
Keywords: Imidazolidine-2-thione, Imidazole-2-thione,1,3-Thiazolidinone, Benzimidazole, Acid hydrazide,1,3- Oxadiazole, Antioxidant, Antibacterial, Antifungal
Cite this paper: Asmaa S. Salman, Anhar Abdel-Aziem, Mrwa J. Alkubbat, Design, Synthesis of Some New Thio-Substituted Imidazole and Their Biological Activity, American Journal of Organic Chemistry, Vol. 5 No. 2, 2015, pp. 57-72. doi: 10.5923/j.ajoc.20150502.01.
Article Outline
1. Introduction
- In general, imidazoles is important family of heterocyclic compounds with a broad interest due to their bioactive properties [1], such as antimicrobial [2-5], antitumor [6, 7], anti- HIV [8], anticonvulsant [9], antitubercular [10], antiprotonzoal [11] and anti-inflammatory [12]. Imidazole ring is also present in some of the clinically used drug structures (asetomidate, cimetidine, omeprazole, lansoprazole, azomycine, azomycine, flumazenil, thyroliberin, methimazole, pilocarpine and etomidate) acting as a pharmacophoric group or a substituent [13]. On other hand, imidazole-2-thione and 2-thioxo-imidazolidin -4-one are present in compounds that have anti-inflammatory [14], antivirala activity [15], antibacterial, antifungal, antioxidant [16, 17], anticancer [18] and anti- proliferative [19]. In view of the important biological properties of the imidazole ring we planned to synthesize some new 2-thio-substituted-imidazoles and imidazolidine derivatives bearing side chains with different structures, as such derivatives could possess interesting and useful biological properties.
2. Experiment
2.1. General
- All melting points were determined in open glass capillaries on a Gallenkamp apparatus and are uncorrected. IR spectra (cm−1) were recorded on a Pye-Unicam spectrophotometer type 1200 using KBr discs. 1H-NMR spectra were recorded on a Varian EM-390 (90MHz) spectrometer using TMS as an internal standard and DMSO-d6 as a solvent. Chemical shifts were expressed in δ (ppm) values. Mass spectra were determined on Finnigan Incos500 (70 ev). Elemental analyses were determined using a Parkin-Elmer 240 C. Microanalyzer. The microanalyses were performed at the Microanalytical Unit, Faculty of Science, Cairo University.
2.2. Chemistry
- 2.2.1. 2-(Benzylthio)-3-(4-chlorophenyl)-5,5-diphenyl- imidazolidin-4-one 3a A mixture of 1 (0.01 mol) and benzyl chloride (0.01 mol) in ethanol (20 ml) was refluxed in the presence of few drop of TEA for 6 h. The solid product separated from the hot mixture was filtered off, washed with water and recrystallized from ethanol to gives 3a as white needles Yield: 75%. m.p.176-178°C. IR (KBr, cm−1): 3029, 2933 (CH), 1731 (CO),1598(C= N).1H-NM R (DMSO-d6) δ ppm: 4.38(s,2H,CH2),7.11-7.47(m,19H,Ar-H).MS,m/z % ,468 (M+, 5.79), 363 (1.02),209 (0.58), 302(22.3), 91(100).Anal. Calcd. for C28H21ClN2OS (468.99):C,71.71; H, 4.51; Cl, 7.56 ; N, 5.97; S,6.84. Found: C, 71.51; H, 4.31; Cl, 7.36 ; N, 5.67; S,6.64 .2.2.2. 2-(Methylthio)-3-(4-chlorophenyl)-5,5-diphenyl- imidazolidin- 4-one 3bTo a solution of compound 1 (0.01 mol) in ethanol (20 ml) and sodium hydroxide (0.01mol in water 2 ml), methyl iodide (0.01mol) was added drop wise. After stirring for 3 h at room temperature (20–23°С), solid products was filtered off washed with water, and recrystallized from ethanol to give 3b as white plates. Yield: 70%.m.p.186-189°C. (KBr, x cm-1): 3054,2918(CH), 1690(CO).1H-NMR (DMSO- d6) δ ppm: 2.59(s,3H,CH3), 7.18-7.47 (m,14H, Ar-H).MS, m /z %, 392(M+,0.50),3.77(26.57).376(100),361(2.15), 233 (85.24), 111(58.26).Anal. Calcd. for C22H17ClN2OS(392.90): C, 67.25; H,4.36 ;Cl,9.02; N,7.13; S,8.16.Found: C, 67.05; H, 4.26 ; Cl,9.00; N,7.00; S, 8.00.2.2.3. Ethyl [3-(4-chlorophenyl)-4-oxo-5,5-diphenyl-imidazolidin-2-ylthio]acetate 4A mixture of 1 (0.01 mol), ethyl chloroacetate (0.012 mol), and anhydrous K2CO3 (0.01 mol) in 40 ml of dry acetone was refluxed on a water bath for 15 h., then poured into cold water. Solid obtained was filtered off, washed with cold water and rerystallized from ethanol to give 4 as pink needles. Yield 50% .m.p.128-130℃. IR (KBr,cm-1) 1741, 1676(2C=O),3055,2984,2918(CH). 1H-NMR(DMSO- d6) δ ppm:1.18 (t,3H, J=7.1 Hz,CH2-CH3), 4.12(q,2H, J=7.1 Hz, CH2CH3),4.04(s, 2H, CH2), 7.16-7.50 (m, 14H, Ar-H). MS, m/z%, 464 (M+, 28.87) , 434(58), 420 (56.70), 352 (96.91), 342 (59.79) 204 (100). Anal. Calcd.for C25H21ClN2O3S (464.96): C,64.58; H,4.55; Cl, 7.62; N, 6.02; S,6.90. Found: C, 64.38; H, 4.45; Cl,7.42; N, 6.00 ; S,6.60. 2.2.4. 2-(Benzoylmethylthio)-3-(4-chlorophenyl)-5,5-di- pheny- l-imidazolidin-4-one 5To a mixture of 1 (0.01 mol) and phenacyl bromide (0.01 mol) in ethanol (30 ml) was added few drops of TEA. The reaction mixture was refluxed for 6h.The solid product separated from the hot mixture was filtered off, washed with water and recrystallized from ethanol to give 5 as yellow plates.Yield: 80%. m.p.196-197℃. IR (KBr, cm-1): 1693, 1671(C=O), 3057, 2906 (CH).1H-NMR (DMSO-d6)δ ppm: 4.10(s,2H,CH2),7.14-7.72 (m,17H, Ar-H), 8.03 (d, 2H, J= 8.7 Hz, Ar-H ).MS, m/z %, 497 (M+,8.82), 420 (8.82), 363 (9.39), 352 (14.94), 77(100). Anal. Calcd. for C29H21 Cl N2O2S(497.01): C,70.08; H,4.26;Cl, 7.13; N,5.64; S, 6.45. Found:C, 69.00; H,4.16;Cl ,7.03 ; N,5.54; S, 6.25.2.2.5. 3-(4-Chlorophenyl)-2- (3-oxo-1,3- diphenylprop -1- en-2-ylthio)-5,5-diphenyl- imidazolidin-4-one 6A mixture of 5 (0.01 mol) and benzaldehyde (0.01 mol) in ethanol (30 ml) containing few drops of piperidine (0.5 ml) was refluxed for 3h. The reaction mixture was left to cooled then poured onto ice water containing few drops of HCl and the obtained solid was crystallized from ethanol to gives 6 as yellow granules.Yield:55%.m.p.260-262℃. IR (KBr,cm-1): 3056, 2920, 2853(CH), 1696, 1663 (C=O).1H- NMR (DMSO-d6) δ ppm:7.09-7.84(m,25H, Ar-H and =CH). Anal.Calcd. for C36H25Cl N2O2S ( 585.11): C,73.90; H,4.31; Cl,6.06; N,4.79; S,5.48.Found: C,73.60; H,4.11; Cl,6.00; N, 4.59; S,5.28.2.2.6. Methyl 2-[3-(4-chlorophenyl)-4-oxo-5,5-diphenyl- imidazolidin-2-ylthio]-3-oxo-3-phenyl-propane-dithioate 8 and Ethyl [2-(3-(4-chlorophenyl)-4-oxo-5, 5-diphenyl- imidazolidin-2-ylthio)-3-oxo-3-phenyl -propane dithioyl) acetate 9 To a cold suspension of KOH (0.01 mol) in dry DMF (25 ml), compound 5 (0.01mol) was added and then carbon disulphide (0.01mol) was added slowly drop wise under stirring over a period of 15 min while the temperature of the mixture was maintained at 5–10℃. The mixture was stirred at room temperature for 12 h, then cooled again to 0℃, methyl iodide (0.01mol) or ethyl chloroacetate (0.01mol) was added drops wise over a period of 10 min. The reaction mixture was stirred for 6 h. at room temperature and then poured into ice cold-water. The resulting precipitate was filtered off, dried and crystallized from proper solvent. 8: Yellow crystals. Yield 60%. m.p. 210-212℃ (DMF- ethanol); IR( KBr,cm-1):3058, 2923, 2851 (CH), 1735,1695 (C=O),1260(C=S);1H-NMR (DMSO-d6) δ ppm:4.32 (s,1H, CH), 2.07(s,3H,CH3);7.13-7.58 (m,19H, Ar-H ).Anal. Calcd. for C31H23ClN2O2S3(587.17):C,63.41; H,3.95; Cl, 6.04; N , 4.77; S, 16.38. Found: C,63.21; H, 3.65; Cl,6.00; N,4.57; S, 16.18.9: Yellow plates. Yield 65%. m.p.140-142oC (benzene). IR (KBr,cm-1): 3059, 2980, 2924 (CH),1695,1741,1633 (C= O), 1291(C=S).1H-NMR(DMSO-d6)δ ppm:4.51(s,1H,CH), 4.04 (s,2H,CH2);1.16(t,3H, J = 7.1 Hz , CH2 CH3) ; 4.10 (q, 2H, J =7.1 Hz, CH2CH3); 7.13- 7.59 (m,17H, Ar-H),8.03 (d, 2H, Ar-H).Anal.Calcd. for C34H27ClN2O4S3 (659.24): C, 61.94; H, 4.13; Cl,5.38; N,4.25; S,14.59. Found:C,61.64; H, 4.00; Cl, 5.28; N,4.05; S,14.29.2.2.7. 2-[1-(3-(4-Chlorophenyl)-4-oxo-5,5-diphenyl-imidazolidin-2-ylthio)-2-oxo-2-phenylethylidene] -3-phenyl-1, 3-thiazolidin-4-one (11)To a stirred suspension of finely powdered KOH (0.01 mol) in dry DMF (10 ml),compound 5 (0.01 mol) was added, and then phenyl isothiocyanate (0.01mol) was added slowly. After complete addition, the mixture was stirred at room temperature for 12 h, then ethyl chloroacetate (0.01 mol) was added to the mixture.The reaction mixture was stirred for 6 h.,then poured into crushed ice. The resulting precipitate was filtrated off, dried and recrystallized from ethanol to give 11 as yellow crystals. Yield 63%. m.p.123- 126℃. IR (KBr cm−1): 1728, 1663,1632 (C=O), 3057, 2979, 2925(CH). 1H-NMR (DMSO-d6) δ ppm: 4.04 (s,2H, CH2), 7.16-7.58 (m,24H, Ar-H). Anal. Calcd. for C38H26ClN3 O3S2 (672.21): C,67.90;H,3.90; Cl,5.27; N, 6.25 ; S,9.54. Found: C, 67.60; H,3.70; Cl,5.07; N,6.05; S,9.24 .2.2.8. 2-[5-Amino-4-(1H-benzoimidazol-2-yl)-3- phenyl -thiophen-2-ylthio]-3-(4-chlorophenyl)-5,5-diphenyl-imidazolidin-4-one 12To a solution of compound 5 (0.01 mol) in absolute ethanol (25 ml) containing morpholine (0.2 ml), 2-(1H- benzimidazol-2-yl)acetonitrile (0.01mol) and elemental sulfur (0.01 mol) were added. The reaction mixture was refluxed for 4 h, then cooled, poured onto ice cold water and neutralized by dilute HCl. The solid so formed was collected by filtration and crystallized from ethanol to give compound 12as brown granules.Yield 65%. m.p.350-352℃. IR(KBr,cm−1):3245,3145,3130(NH,NH2), 1695(C=O), 3045, 2907 ( CH ).1H-NMR (DMSO-d6) δ ppm: 4.81 (s, 2H,NH2), 13.03 (s,1H,NH), 7.13-7.58 (m,23H, Ar-H). Anal. Calcd.for C38H26ClN5OS2( 668.23):C,68.30; H,3.92; Cl, 5.31; N,10.48; S, 9.60.Found: C,68.00; H,3.62; C, 5.11; N,10.28; S ,9.40 2.2.9. General procedure for synthesis of 1-(4-substituted phenyl)-4,5-diphenyl-S-alkyl-imidazole13a-d and 15a-eA mixture of 2a,b ( 0.01 mol) and respective halo compounds (0.01 mol) in ethanol (50 ml) was refluxed in the presence of few drops of TEA for 6-8 h. the solid product separated from the hot mixture was filtered off, washed with water and recrystallized from the proper solvent.2-(Benzylthio)-1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazole 13aWhite crystals. Yield: 55%. m.p.180-182°C (ethanol- DMF).IR(KBr,cm−1):3030,2927(CH),1592(C=N).1H- NMR (DMSO-d6) δ ppm:4.38 (s,2H, CH2),7.10-7.49(m,19H, Ar- H). MS, m/z %, 453( M+, 18.49) , 452( 16.63), 417(20.45), 362 (2.17), 302(100),304 (34.77) ,91 (52.89). Anal Calcd. for C28H21ClN2S (452.99): C,74.24;H,4.67; Cl,7.83; N, 6.18; S, 7.08. Found: C,74.01;H,4.37;Cl,7.53; N,6.08; S, 6.81.2-(Benzythio)-1-(4-nitrophenyl)-4,5-diphenyl-1H-imidazole 13bYellow powder. Yield: 75 %. m.p.160-162°C (ethanol- DMF).IR(KBr,cm−1): 3063,2917(CH),1592(C=N).1H-NMR (DMSO-d6) δ ppm:4.40(s,2H,CH2),6.63-8.18(m,19H,Ar-H) MS, m/z %,464 (M++1, 27.22),463 (M+,77.39), 430 (43.38), 372(5.79),309 (43.34),91(100).Anal. Calcd. for C28H21N3O2 S: (463.55) C,72.55; H,4.57; N,9.06; S,6.92.Found: C,72.25; H, 4.37; N,9.00; S,6.62.2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]acetonitrile 13cPal brown granules.Yield:60%. m.p 190-192°C (ethanol). IR(KBr,cm−1): 2246(CN), 3026, 2988, 2862(CH),1599 (C=N). 1H-NMR (DMSO-d6) δ ppm:4.28(s,2H, CH2),7.19- 7.51(m,14H, Ar-H). MS, m/z % :401(M+,38.96), 361 (3.58) , 303 (100),103 (16.42),79 (86.37).Anal. Calcd. for C23H16Cl N3S (401.91):C, 68.73; H, 4.01; Cl, 8.82; N,10.46; S, 7.98. Found:C,68.53;H,3.89; Cl, 8.55; N,10.16.S,7.962-[4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol-2-ylthio]-acetonitrile 13dYellow plates. Yield 61%. m.p. 176-178°C (ethanol-DMF). IR (KBr, cm−1):2240 (CN), 3073, 2973, 2921 (CH), 1594 (C=N).1H-NMR (DMSO-d6) δ ppm: 4.29(s, 2H,CH2), 6.62- 8.27(m,14H,Ar-H). MS, m/z %, 412 (M+, 49.12), 413 (M++1,14.81),372 (15.46),314 (100),76 (28.30). Anal. Calcd. for C23H16N4O2S(412.46).C,66.97; H,3.91; N,13.58; S,7.77. Found: C, 66.67; H,3.61; N,13.32; S,7.57.1-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]propan-2-one 15aPale yellow granules.Yield:70%.m.p.181-183°C (ethanol). IR(KBr,cm−1):1711(C=O), 3049, 2974, 2947 (CH). 1H- NMR (DMSO- d6) δ ppm: 2.30(s,3H, COCH3), 4.17(s,2H,S CH2),7.18-7.50 (m,14H, Ar-H).M.S,m/z %:418 (M+, 52.25) , 383(46.85),292(50.45), 264 (66.67), 153 (45.05), 77 (40.54), 54 (100). Anal.Calcd. for: C24H19ClN2OS (418.93):C,68.81; H, 4.57;Cl,8.46; N,6.69;S,7.65.Found: C,68.51; H,4.27;Cl, 8.26 ; N,6.39; S,7.35.1-[4,5-Diphenyl-1-(4-nitrophenyl )-1H-imidazol-2-ylthio]propan-2-one 15bYellow crystals. Yield: 65%. m.p.150-152°C (ethanol–DMF). IR (KBr, cm−1):1680 (C=O), 3062, 2950, 2915, 2859 (CH).1H-NMR (DMSO-d6) δ ppm:2.30 (s,3H,COCH3), 4.20 (s, 2H, SCH2), 6.63-8.16 (m,14H, Ar-H). MS, m/z%: 430 (M+ +1, 15.36), 429 (M+,48.06),386 (100), 372 (2.29), 340 (20.31),314(34.42),76 (27.29). Anal. Calcd. for: C24H19N3O3S (429.49): C,67.12; H,4.46; N, 9.78; S,7.47. Found: C, 67.02 ; H,4.16; N,9.48; S,7.17.2-[Benzoylmethylthio]-1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazole 15cWhite plates. Yield: 75%. m.p. 216-218°C (ethanol). IR(KBr, cm−1): 1691(C=O),3055, 2952(CH);1H-NMR (DMSO -d6) δ ppm:4.81(s,2H,SCH2),7.13–8.06 (m,19H, Ar-H), MS, m/z %: 481(M+,35.85), 366 (38.9), 293 (32.70), 264(49.06), 76(37.74),63.95(100). Anal. Calcd. for: C29H21ClN2OS(481.0): C,72.41; H,4.40; Cl,7.37; N,5.82; S,6.67. Found: C,72.11; H,4.10; Cl, 7.17; N, 5.52;S,6.37.2-[(Benzoylmethyl)thio]-4,5-diphenyl-1-(4-nitrophenyl)- 1H-imidazole 15dYellow crystals.Yield:72%. m.p.170-174°C (ethanol). IR (KBr,cm−1):1683(C=O),3062,2988(CH). 1H-NMR (DMSO-d6) δ ppm: 4.84(s,2H,SCH2), 6.90–8.24(m,19H, Ar-H).MS, m/z %, 492 (M++1,16.67), 491(M+,47.37), 461 (55.62), 432 (47.37), 419(48.25), 369(50.88), 292 (50), 225 (100). Anal. Calcd. for:C29H21N3O3S(491.561): C,70.86; H,4.31; N, 8.55; S ,6.52.Found:C,70.56; H,4.01; N,8.25;S,6.22.2-[4,5-Diphenyl-1-(4-nitrophenyl )-1H-imidazol-2-ylthio]acetamide 15eYellow brownish needles. Yield: 65%. m.p.160-163°C (ethanol). IR(KBr,cm−1):1676(C=O),3439,3379(NH2), 3058, 2918(CH).1H-NMR (DMSO-d6) δ ppm:3.93 (s,2H,SCH2), 6.64-8.27(m,16H,Ar-H and NH2).M.S, m/z %:430 (M+, 43.78),414(26.73), 369(30.41),362 (32.35),250 (26.73), 152 (8.76),8 (100). Anal. Calcd. For: C23H18N4O3S (430.48):C, 64.17; H,4.21; N,13.01; S,7.45. Found: C,64.00; H,4.00; N, 13.00 ; S,7.15.2.2.10. 1-(4-Chlorophenyl)-2-(methylthio)-4,5-diphenyl- 1H-imidazole 16To a solution of compound 2a (0.01mol) in ethanol (20ml) and sodium hydroxide (0.01mol in water 2 ml), dimethyl sulfate (0.01mol) was added drop wise. The reaction mixture was refluxed for 6 h. The reaction mixture was left to cool, the crude solid was filtered off,washed with water, and recrystallized from ethanol to give 16 as pale yellow powder. Yield 67%. m.p.190-193°C. IR (KBr, cm−1): 3055, 2920( CH), 1594(C=N).1H-NMR (DMSO-d6) δ ppm: 2.61 (s, 3H, CH3),7.14-7.48 (m, 14H, Ar-H). MS, m/z %, 377 (M+, 40.60), 376 (28.97), 375 (100), 341(1.92), 302 (24.65), 265.9(3.50),192(66.4).Anal.Calcd.for:C22H17ClN2S(376.90): C,70.11; H,4.55; Cl, 9.41; N,7.43; S,8.51.Found:C,70.00; H, 4.25; Cl,9.21; N,7.13; S,8.31.2.2.11. General procedure for synthesis of ethyl [4,5- diphenyl-1-(4-substituted phenyl )-1H-imidazol-2-ylthio] acetate 17a,bA mixture of 2a,b (0.01 mol) and ethyl chloroacetate (0.01mol)in 15ml ethanol containing few drops of TEA was refluxed for 6 h. The reaction mixture was left to cool, the crude solid was filtered off, washed with water and recry- stallized from ethanol.Ethyl (1-(4-chlorophenyl)-4,5-diphenyl-1H-imidazol-2- ylthio) acetate 17aWhite crystals.Yield:55%. m.p.128-130°C (ethanol). IR (KBr,cm−1):1740(C=O), 3054,2920(CH). 1H-NMR(DMSO- d6) δ ppm:1.17(t,3H, J=7.1 Hz, CH2
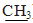 );4.02 (s,2H,CH2); 4.11 (q, 2H, J=7.1 Hz,
);4.02 (s,2H,CH2); 4.11 (q, 2H, J=7.1 Hz, 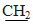 CH3);7.17-7.49 (m,14H,Ar-H). MS, m/z %,448 (M+,100),449 (M++1,31.63), 422.9 (0.69), 375 (55.44), 362(5.22). Anal. Calcd. for: C25H21ClN2O2S (448.96): C,66.88; H,4.71; Cl,7.90; N,6.24; S,7.14. Found : C, 66.58;H,4.31; Cl,7.60; N, 6.00; S,7.00. Ethyl (4,5-diphenyl-1-(4-nitrophenyl)- 1H-imidazol-2-yl- thio) acetate 17bYellow powder .Yield:50 %.m.p.144-146°C (ethanol); IR (KBr, cm−1): 1734(C=O), 3074, 2982, 2926(CH); 1H-NMR (DMSO-d6) δ ppm:1.18(t,3H, J=7.1 Hz CH2
CH3);7.17-7.49 (m,14H,Ar-H). MS, m/z %,448 (M+,100),449 (M++1,31.63), 422.9 (0.69), 375 (55.44), 362(5.22). Anal. Calcd. for: C25H21ClN2O2S (448.96): C,66.88; H,4.71; Cl,7.90; N,6.24; S,7.14. Found : C, 66.58;H,4.31; Cl,7.60; N, 6.00; S,7.00. Ethyl (4,5-diphenyl-1-(4-nitrophenyl)- 1H-imidazol-2-yl- thio) acetate 17bYellow powder .Yield:50 %.m.p.144-146°C (ethanol); IR (KBr, cm−1): 1734(C=O), 3074, 2982, 2926(CH); 1H-NMR (DMSO-d6) δ ppm:1.18(t,3H, J=7.1 Hz CH2 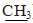 );4.06 (s, 2H, CH2) ; 4.13(q, 2H, J=7.1Hz,
);4.06 (s, 2H, CH2) ; 4.13(q, 2H, J=7.1Hz, 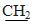 CH3 );7.21–8.28( m, 14H,Ar-H).MS, m/z %,459 (M+,94.15),430 (1.76), 414 (8.35) , 413 (1.53), 372 (6.28), 86 (100), Anal. Calcd. for: C25H21N3O4S(459.51):C,65.34;H,4.61;N,9.14;S,6.98. Found: C, 65.04; H,4.31; N, 9.00 ; S,6.68.2.2.12. 2-(4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol-2- ylthio)acetohydrazide 18A mixture of compound 17b (0.01 mol) and hydrazine hydrate (10 ml, 85%) in ethanol (20 ml) was stirred well and refluxed for 6 h. The reaction mixture was cooled and the crude product was collected by filtration, washed with water and recrystallized from ethanol to give 18 as yellow plates. Yield: 60%.m.p. 254 - 258°C. IR(KBr, cm−1): 1664(C=O), 3394,3331,3231(NH,NH2),3055,2923(CH);1616(C=N).1H-NMR (DMSO - d6) δ ppm:6.41 - 7.43 (m,14,Ar-H), 12.80(s,1H,NH), 5.34(s,2H,NH2), 3.85(s,2H,CH2). MS, m/z%, 443 (M+-2, 81.42), 398 (60.03),323 (71.68), 292 (72.57),92 (100). Anal.Calcd for C23H19N5O3S(445.49): C,62.01; H, 4.30;N,15.72;S,7.20. Found: C,61.89; H,4.00;N, 15.52;S, 7.00.2.2.13. 2-[4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol-2- ylthio]-N'-benzylidene-acetohydrazide 19A mixture of acid hydrazide 18 (0.01 mol) and benzaldehyde (0.01 mol) in absolute ethanol (30 ml) was heated under reflux for 4 h in presence of TEA (0.2 ml). Reaction mixture was then poured into ice cold water and filtered off and recrystallized from DMF to give 19 as white plates Yield: 65% .m.p. 288-290℃. IR ( KBr, cm−1): 1658(C=O), 3226 (NH),3049,2920 (CH).1H-NMR(DMSO- d6) δ ppm: 5.01 (s, 2H,CH2),6.32–6.44(m,3H,Ar-H and CH= C), 6.76- 6.82 (m, 2H,Ar-H),7.13-7.38 (m,15H, Ar- H),12.00(s, 1H, NH). Anal. Calcd. for C30H23N5O3S (533.60): C,67.53; H, 4.34; N,13.12; S,6.01.Found: C,67.33; H,4.24;N, 13.00;S , 5.89 . 2.2.14. 1-[2-(4,5-Diphpenyl-1-(4-nitrophenyl)-1H-imidazol- 2-ylthio)acetyl]-4-phenyl-thiosemicarbazide 20A mixture of acid hydrazide 18 ( 0.01mol ) and phenyl isothiocyanate (0.01mol) was refluxed in ethanol for 4h. The reaction mixture was left to cooled, the crude solid was filtered off,washed twice with cold ethanol, dried and recrystallized from ethanol to give 20 as white powder. Yield 62%. m.p.158-160℃. IR (KBr,cm−1 ):3428,3212 (NH), 3052, 2923(CH), 1664 (C=O),1251(C=S).1H-NMR (DMSO- d6) δ ppm:4.17( s,2H,CH2 ),12.96 (s,1H,NH), 12.80 (s, 1H, NH),12.48(s,1H, NH), 6.42-7.83(m,19H, Ar-H).Anal.Calcd. for C30H24N6O3S2(580.68):C,62.05;H,4.17;N,14.47;S, 11.04. Found: C,61.99 ;H ,4.00; N,14.27; S,11.0 . 2.2.15. 2-(4,5-Diphpenyl-1-(4-nitrophenyl)-1H-imidazol- 2-ylthio)-N'-(4-oxo-3-phenyl-thiazolidin-2-ylidene)-acetohydrazide 21To a suspension of thiosemicarbazide derivatives 20 (0.01mol) in glacial acetic acid (5 ml), anhydrous sodium acetate (0.02 mol) and chloroacetic acid (0.02 mol) were added. The reaction mixture was refluxed for 4 h then cooled, diluted with water and allowed to stand overnight. The product was filtered off and recrystallised from ethanol to give 21 as white plates. Yield: 55%. m.p.139-140℃. IR (KBr, cm−1):3374(NH), 1724, 1671(C= O).1H-NMR (DMSO - d6) δ ppm: 4.33(s,2H,CH2),4.50 (s, 2H, CH2), 11.18 (s,1H, NH), 6.43-7.62(m,19 H, Ar-H). Anal. Calcd for C32H24 N6O4 S2(620.70):C,61.92;H,3.90;N,13.54;S,10.33. Found:C, 61.62; H, 3.70; N, 13.34; S,10.13.2.2.16. 5-[ 4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol - 2-ylthio )methyl]-1,3,4-oxadiazole-2( 3H)thione 22A solution of acid hydrazide 18 (0.01 mol) in pyridine (50 ml) and carbon disulphide (0.02 mol) was refluxed on water bath for 7h.The reaction mixture was allowed to cooled and then acidified with dilute hydrochloric acid. The solid obtained was collected by filtration, washed with water,and recrystallized from ethanol to give 22 as white granules. m.p. 278-280°C. IR (KBr, cm−1):3430 (NH), 3053, 2919(CH), 1623(C=N). 1H-NMR(DMSO-d6):δ ppm:12.79 (s, 1H,NH), 6.41(d,2H, J= 8.4,Ar-H),6.79 (d.2H, J= 8.4, Ar- H), 7.14-7.34 (m, 10H, Ar-H),5.19 ( s,2H,CH2).Anal.Calcd for C24H17N5O3S2 (487.55): C,59.12; H,3.51; N ,14.36; S, 13.15. Found: C, 59.00; H,3.31;N ,14.06 ;S,13.00 .2.2.17. General procedure for Synthesis of N-chloro- acetamide derivatives 24a-c Chloroacetyl chloride (0.05 mol) was added drops wise over one hour to a solution of suitable aryl amine and /or appropriate sulfa drug (0.05 mol) in ethanol / DMF (50 ml, 1:1) contain few drops of TEA. Then the solution was left to stirring overnight. The resulting precipitates was filtered off and washed with ethanol and used in second step. 2.2.18. General procedure for synthesis of 2-(1H-imidaz- ol-2-ylthio)-N-substituted acetamide derivatives 25a-eA mixture of 2a,b (0.01 mol) and N-chloroarylacetamide derivatives 24 a-c (0.01 mol) in DMF (25 ml) contain few drops of TEA was heated under reflux for 6h. The reaction mixture was left to cool and then poured to ice cooled water (100 ml). The solid product that formed was filtered off, dried well and recrystallized from suitable solvent.2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-N-p-tolyl-acetamide 25aPall yellow powder. Yield: 70%. m.p.158-160°C (ethanol). IR (KBr, cm−1):3246 (NH ),1683 (C=O),3041, 2931(CH), 1600(C=N).1H-NMR(DMSO-d6) δ ppm: 4.11 (s, 2H, CH2), 2.24(s, 3H, CH3), 10.36 (s,1H, NH), 7.09 -7.47(m,18H, Ar-H). MS, m/z %, 510 (M+, 26.25), 404 (17.60), 375(27.06), 362(8.612), 193 (100), 147 (10.5). Anal. Calcd. for:C30H24ClN3OS(510.05):C,70.64; H, 4.74; Cl, 6.95; N,8.24; S,6.29 .Found C,70.44; H,4.54; Cl, 6.65; N,8.04; S,6.09 .2- [4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol-2-ylthio] -N-p-tolyl-acetamide ( 25b )White needles. Yield: 75%. m.p.168-170°C (ethanol- DMF). IR(KBr,cm−1): 3246(NH),1684(C=O),1599(C=N). 1H-NMR (DMSO- d6) δ ppm: 4.10( s, 2H, CH2), 2.24 (s, 3H, CH3), 10.31(s,1H, NH),7.09-7.47(m,18H, Ar-H). MS, m/z %, 518(M+-2,40.80), 474 (19.54), 459(10.92), 399 (37.93), 385(32.76),342(100), 306(24.71), 148(12.64),134 (14.94). Anal. Calcd. for:C30H24N4O3S(520.603):C, 69.21; H,4.65; N,10.76;S,6.16.Found:C,69.00; H,4.45; N,10.46; S,6.00.N-(4-Aminosulfonylphenyl)-2-[1-(4-chlorophenyl)-4.5- diphenyl-1H- imidazol-2-ylthio]acetamide ( 25c )White granules. Yield: 60%. m.p.> 360°C (ethanol).IR(KBr, cm−1): 1670 (C=O),1593(C=N),3330,3250,3150 (NH,NH2),3048,2913(CH).1H-NMR (DMSO-d 6) δ ppm: 4.11(s,2H,CH2),13.04(s,1H, NH),7.12-7.48(m,20H, Ar-H and NH2).MS, m/z % ,575 (M+,63.06), 559 (51.35), 523 (49.55), 383(100), 362 (29.73), 212(49.55).Anal. Calcd. for C29H23ClN4O3S2 (575.10):C, 60.56; H,4.03; Cl, 6.16; N , 9.74; S, 11.15. Found: C,60.26; H,4.00; Cl,6.00 ; N , 9.53; S, 11.00.N-(4-Aaminosulfonylphenyl)-2-(4.5-diphenyl-1-(4-nitrophenyl)-1H-imidazol-2-ylthio)acetamide (25d)White plates. Yield: 68%. m.p.280-282°C (ethanol); IR (KBr,cm−1): 1668(C=O),1594(C=N),3375, 3292, 3140 (NH,NH2),3056,2913(CH).1H-NMR(DMSO-d6) δ ppm: 4.12 (s,2H, CH2), 13.16 (s,1H, NH),6.91-8.21(m, 20H, Ar-H and NH2).M.S, m/z % ,585(M+, 48.65),523 (84.68), 504(73.87), 448(59.46), 372(66.67), 214(61.26), 143 (100). Anal. Calcd. for C29H23N5O5S2(585.65): C, 59.47; H, 3.96; N,11.96; S,10.95.Found: C,59.27; H,3.66; N, 11.73; S, 10.65 .2-[4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol- 2-ylthio]-N-[4-(pyrimidin-2-ylsulfamoyl)-phenyl]- acetamide 25eGray powder.Yield:55%.m.p.238-240°C (ethanol). IR (KBr, cm−1):1659(C=O), 3061,2966(CH),3238,3181(NH). 1H-NMR (DMSO-d6) δ ppm: 4.13 (s,2H, CH2), 10.83 (s, 1H,NH),11.60(s, 1H,NH), 7.13- 7.48 (m, 21H, Ar-H and pyrimidine). MS, m/z %, 663(M+ ,17.76),584 (18.07) , 569 (22.74),506(21.18),429 (35.20), 371 (20.56), 291 (21.81), 276(16.82),248(17.13), 57(100). Anal. Calcd. for C33H25 N7O5S2(663.72):C,59.72;H,3.80; N,14.77; S;9.66. Found: C, 59.52; H,3.60; N,14.67; S;9.46.
CH3 );7.21–8.28( m, 14H,Ar-H).MS, m/z %,459 (M+,94.15),430 (1.76), 414 (8.35) , 413 (1.53), 372 (6.28), 86 (100), Anal. Calcd. for: C25H21N3O4S(459.51):C,65.34;H,4.61;N,9.14;S,6.98. Found: C, 65.04; H,4.31; N, 9.00 ; S,6.68.2.2.12. 2-(4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol-2- ylthio)acetohydrazide 18A mixture of compound 17b (0.01 mol) and hydrazine hydrate (10 ml, 85%) in ethanol (20 ml) was stirred well and refluxed for 6 h. The reaction mixture was cooled and the crude product was collected by filtration, washed with water and recrystallized from ethanol to give 18 as yellow plates. Yield: 60%.m.p. 254 - 258°C. IR(KBr, cm−1): 1664(C=O), 3394,3331,3231(NH,NH2),3055,2923(CH);1616(C=N).1H-NMR (DMSO - d6) δ ppm:6.41 - 7.43 (m,14,Ar-H), 12.80(s,1H,NH), 5.34(s,2H,NH2), 3.85(s,2H,CH2). MS, m/z%, 443 (M+-2, 81.42), 398 (60.03),323 (71.68), 292 (72.57),92 (100). Anal.Calcd for C23H19N5O3S(445.49): C,62.01; H, 4.30;N,15.72;S,7.20. Found: C,61.89; H,4.00;N, 15.52;S, 7.00.2.2.13. 2-[4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol-2- ylthio]-N'-benzylidene-acetohydrazide 19A mixture of acid hydrazide 18 (0.01 mol) and benzaldehyde (0.01 mol) in absolute ethanol (30 ml) was heated under reflux for 4 h in presence of TEA (0.2 ml). Reaction mixture was then poured into ice cold water and filtered off and recrystallized from DMF to give 19 as white plates Yield: 65% .m.p. 288-290℃. IR ( KBr, cm−1): 1658(C=O), 3226 (NH),3049,2920 (CH).1H-NMR(DMSO- d6) δ ppm: 5.01 (s, 2H,CH2),6.32–6.44(m,3H,Ar-H and CH= C), 6.76- 6.82 (m, 2H,Ar-H),7.13-7.38 (m,15H, Ar- H),12.00(s, 1H, NH). Anal. Calcd. for C30H23N5O3S (533.60): C,67.53; H, 4.34; N,13.12; S,6.01.Found: C,67.33; H,4.24;N, 13.00;S , 5.89 . 2.2.14. 1-[2-(4,5-Diphpenyl-1-(4-nitrophenyl)-1H-imidazol- 2-ylthio)acetyl]-4-phenyl-thiosemicarbazide 20A mixture of acid hydrazide 18 ( 0.01mol ) and phenyl isothiocyanate (0.01mol) was refluxed in ethanol for 4h. The reaction mixture was left to cooled, the crude solid was filtered off,washed twice with cold ethanol, dried and recrystallized from ethanol to give 20 as white powder. Yield 62%. m.p.158-160℃. IR (KBr,cm−1 ):3428,3212 (NH), 3052, 2923(CH), 1664 (C=O),1251(C=S).1H-NMR (DMSO- d6) δ ppm:4.17( s,2H,CH2 ),12.96 (s,1H,NH), 12.80 (s, 1H, NH),12.48(s,1H, NH), 6.42-7.83(m,19H, Ar-H).Anal.Calcd. for C30H24N6O3S2(580.68):C,62.05;H,4.17;N,14.47;S, 11.04. Found: C,61.99 ;H ,4.00; N,14.27; S,11.0 . 2.2.15. 2-(4,5-Diphpenyl-1-(4-nitrophenyl)-1H-imidazol- 2-ylthio)-N'-(4-oxo-3-phenyl-thiazolidin-2-ylidene)-acetohydrazide 21To a suspension of thiosemicarbazide derivatives 20 (0.01mol) in glacial acetic acid (5 ml), anhydrous sodium acetate (0.02 mol) and chloroacetic acid (0.02 mol) were added. The reaction mixture was refluxed for 4 h then cooled, diluted with water and allowed to stand overnight. The product was filtered off and recrystallised from ethanol to give 21 as white plates. Yield: 55%. m.p.139-140℃. IR (KBr, cm−1):3374(NH), 1724, 1671(C= O).1H-NMR (DMSO - d6) δ ppm: 4.33(s,2H,CH2),4.50 (s, 2H, CH2), 11.18 (s,1H, NH), 6.43-7.62(m,19 H, Ar-H). Anal. Calcd for C32H24 N6O4 S2(620.70):C,61.92;H,3.90;N,13.54;S,10.33. Found:C, 61.62; H, 3.70; N, 13.34; S,10.13.2.2.16. 5-[ 4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol - 2-ylthio )methyl]-1,3,4-oxadiazole-2( 3H)thione 22A solution of acid hydrazide 18 (0.01 mol) in pyridine (50 ml) and carbon disulphide (0.02 mol) was refluxed on water bath for 7h.The reaction mixture was allowed to cooled and then acidified with dilute hydrochloric acid. The solid obtained was collected by filtration, washed with water,and recrystallized from ethanol to give 22 as white granules. m.p. 278-280°C. IR (KBr, cm−1):3430 (NH), 3053, 2919(CH), 1623(C=N). 1H-NMR(DMSO-d6):δ ppm:12.79 (s, 1H,NH), 6.41(d,2H, J= 8.4,Ar-H),6.79 (d.2H, J= 8.4, Ar- H), 7.14-7.34 (m, 10H, Ar-H),5.19 ( s,2H,CH2).Anal.Calcd for C24H17N5O3S2 (487.55): C,59.12; H,3.51; N ,14.36; S, 13.15. Found: C, 59.00; H,3.31;N ,14.06 ;S,13.00 .2.2.17. General procedure for Synthesis of N-chloro- acetamide derivatives 24a-c Chloroacetyl chloride (0.05 mol) was added drops wise over one hour to a solution of suitable aryl amine and /or appropriate sulfa drug (0.05 mol) in ethanol / DMF (50 ml, 1:1) contain few drops of TEA. Then the solution was left to stirring overnight. The resulting precipitates was filtered off and washed with ethanol and used in second step. 2.2.18. General procedure for synthesis of 2-(1H-imidaz- ol-2-ylthio)-N-substituted acetamide derivatives 25a-eA mixture of 2a,b (0.01 mol) and N-chloroarylacetamide derivatives 24 a-c (0.01 mol) in DMF (25 ml) contain few drops of TEA was heated under reflux for 6h. The reaction mixture was left to cool and then poured to ice cooled water (100 ml). The solid product that formed was filtered off, dried well and recrystallized from suitable solvent.2-[1-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazol-2-ylthio]-N-p-tolyl-acetamide 25aPall yellow powder. Yield: 70%. m.p.158-160°C (ethanol). IR (KBr, cm−1):3246 (NH ),1683 (C=O),3041, 2931(CH), 1600(C=N).1H-NMR(DMSO-d6) δ ppm: 4.11 (s, 2H, CH2), 2.24(s, 3H, CH3), 10.36 (s,1H, NH), 7.09 -7.47(m,18H, Ar-H). MS, m/z %, 510 (M+, 26.25), 404 (17.60), 375(27.06), 362(8.612), 193 (100), 147 (10.5). Anal. Calcd. for:C30H24ClN3OS(510.05):C,70.64; H, 4.74; Cl, 6.95; N,8.24; S,6.29 .Found C,70.44; H,4.54; Cl, 6.65; N,8.04; S,6.09 .2- [4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol-2-ylthio] -N-p-tolyl-acetamide ( 25b )White needles. Yield: 75%. m.p.168-170°C (ethanol- DMF). IR(KBr,cm−1): 3246(NH),1684(C=O),1599(C=N). 1H-NMR (DMSO- d6) δ ppm: 4.10( s, 2H, CH2), 2.24 (s, 3H, CH3), 10.31(s,1H, NH),7.09-7.47(m,18H, Ar-H). MS, m/z %, 518(M+-2,40.80), 474 (19.54), 459(10.92), 399 (37.93), 385(32.76),342(100), 306(24.71), 148(12.64),134 (14.94). Anal. Calcd. for:C30H24N4O3S(520.603):C, 69.21; H,4.65; N,10.76;S,6.16.Found:C,69.00; H,4.45; N,10.46; S,6.00.N-(4-Aminosulfonylphenyl)-2-[1-(4-chlorophenyl)-4.5- diphenyl-1H- imidazol-2-ylthio]acetamide ( 25c )White granules. Yield: 60%. m.p.> 360°C (ethanol).IR(KBr, cm−1): 1670 (C=O),1593(C=N),3330,3250,3150 (NH,NH2),3048,2913(CH).1H-NMR (DMSO-d 6) δ ppm: 4.11(s,2H,CH2),13.04(s,1H, NH),7.12-7.48(m,20H, Ar-H and NH2).MS, m/z % ,575 (M+,63.06), 559 (51.35), 523 (49.55), 383(100), 362 (29.73), 212(49.55).Anal. Calcd. for C29H23ClN4O3S2 (575.10):C, 60.56; H,4.03; Cl, 6.16; N , 9.74; S, 11.15. Found: C,60.26; H,4.00; Cl,6.00 ; N , 9.53; S, 11.00.N-(4-Aaminosulfonylphenyl)-2-(4.5-diphenyl-1-(4-nitrophenyl)-1H-imidazol-2-ylthio)acetamide (25d)White plates. Yield: 68%. m.p.280-282°C (ethanol); IR (KBr,cm−1): 1668(C=O),1594(C=N),3375, 3292, 3140 (NH,NH2),3056,2913(CH).1H-NMR(DMSO-d6) δ ppm: 4.12 (s,2H, CH2), 13.16 (s,1H, NH),6.91-8.21(m, 20H, Ar-H and NH2).M.S, m/z % ,585(M+, 48.65),523 (84.68), 504(73.87), 448(59.46), 372(66.67), 214(61.26), 143 (100). Anal. Calcd. for C29H23N5O5S2(585.65): C, 59.47; H, 3.96; N,11.96; S,10.95.Found: C,59.27; H,3.66; N, 11.73; S, 10.65 .2-[4,5-Diphenyl-1-(4-nitrophenyl)-1H-imidazol- 2-ylthio]-N-[4-(pyrimidin-2-ylsulfamoyl)-phenyl]- acetamide 25eGray powder.Yield:55%.m.p.238-240°C (ethanol). IR (KBr, cm−1):1659(C=O), 3061,2966(CH),3238,3181(NH). 1H-NMR (DMSO-d6) δ ppm: 4.13 (s,2H, CH2), 10.83 (s, 1H,NH),11.60(s, 1H,NH), 7.13- 7.48 (m, 21H, Ar-H and pyrimidine). MS, m/z %, 663(M+ ,17.76),584 (18.07) , 569 (22.74),506(21.18),429 (35.20), 371 (20.56), 291 (21.81), 276(16.82),248(17.13), 57(100). Anal. Calcd. for C33H25 N7O5S2(663.72):C,59.72;H,3.80; N,14.77; S;9.66. Found: C, 59.52; H,3.60; N,14.67; S;9.46.2.3. Biological Evaluation
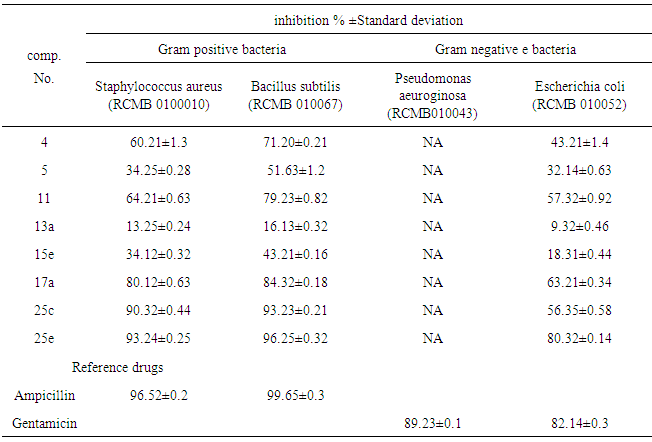
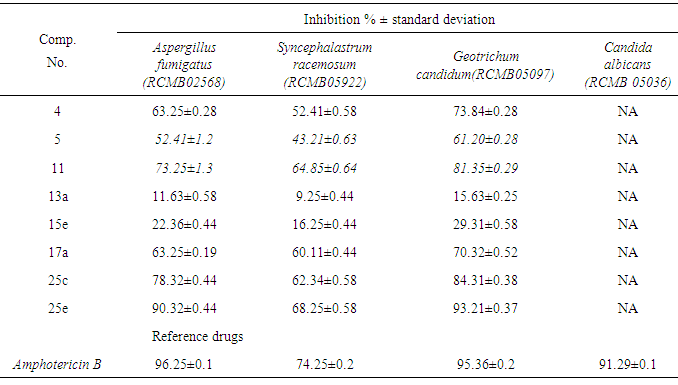
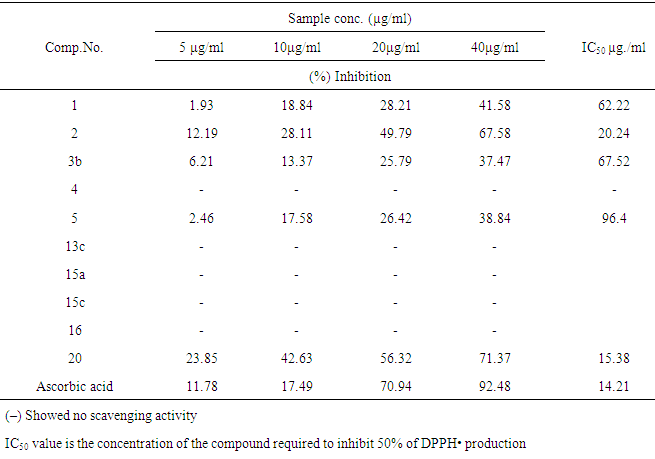
- 2.3.1. Antimicrobial AssaysSynthesized compounds 4,5,11,13a,15e,17a,25c and 25e were screened for their antimicrobial activities in vitro against two species of Gram-positive bacteria, namely Staphylococcus aureus(RCMB 0100010) and Bacillus subtilis (RCMB 010067), two Gram-negative bacteria, namely Pseudomonas aeuroginosa (RCMB010043) and Escherichia coli (RCMB 010052) and against four species of fungi, namely Aspergillus fumigatus (RCMB 02568), Syncephalastrum racemosum (RCMB 05922),Geotrichum candidum (RCMB05097) and Candida albicans (RCMB 05036).The antibacterial and antifungal activity were determined by means of inhibition % ± standard deviation at a concentration of 100 μg/ ml of tested samples[20-22]. Optical densities of antimicrobial were measured after 24 hours at 37℃ to bacteria and measured after 48 hours at 28℃ to fungal using a multidetection microplate reader at the Regional Center for Mycology and Biotechnology (Sun Rise-2.3.2. DPPH Antioxidant AssayThe free radical scavenging activity of the synthesis compounds 1,2, 3b, 4, 5, 13c, 15a, 15c, 16 and 20were evaluated by 1,1-diphenyl-2-picrylhydrazil (DPPH) accord- ing to the previously reported method [23]. Briefly, an 0.1 m M solution of DPPH in methanol was prepared, and 1 ml of this solution was added to 3ml of the solutions of all compound in methanol at different concentrations (5,10, 20, 40 μg / ml).The mixtures were shaken vigorously and allowed to stand at room temperature for 30 min. Then their absorbance were measured at 517 nm using a UV-VIS spectrophotometer (Genesys 10 UV: Thermo Electron Corporation). Ascorbic acid was used as the reference. Lower absorbance values of reaction mixture indicate higher free radical scavenging activity. The capability to scavenge the DPPH radical was calculated by using the following formula :DPPH scavenging effect (% inhibition) = [Ac-As/Ac x100 ]Where Ac is the absorbance of the control (blank, without test sample) and As is the absorbance of the test samples. IC50 value is the concentration of the compound required to inhibit 50% of DPPH• production
3. Results and Discussion
3.1. Chemistry
- The synthetic procedures adopted to obtain the target compounds are depicted in Schemes 1- 4.The starting compounds3-(4-chlorophenyl)-5,5-diphenyl-2-thioxoimidazol- idin-4-one 1 and 1-(4-substituted phenyl)-4,5-diphenyl- 1H- imidazole-2-thione 2a,b were prepared according to the previously reported procedure [24]. Alkylation of 5,5-di- phenyl-2-thioxoimidazolidin-4-one 1 with benzyl chloride and methyl iodide afforded 2-benzylthio- and 2-methylthio- 3-(4-chlorophenyl)-5,5-diphenyl-imidazolidin-4-one 3a,b respectively. The structures of 3a,b was supported on the basis of elemental analyses and spectral data. The 1H-NMR spectra of 3a in (DMSO-d6) revealed a singlet at δ 4.38 ppm assigned to the SCH2 protons. Its mass spectrum showed a molecular ion peak at m/z 468.99 corresponding to a molecular formula C28H21ClN2OS.Imidazolidin-4-one derivative 1 was treated with ethyl chloroacetate in dry acetone containing anhydrous K2CO3 afforded ethyl [3-(4-chlorophenyl)-4-oxo-5,5-diphenyl- imidazolidin-2-ylthio]acetate 4. IR spectra of 4 showed a characteristic absorption bands at 1741 for carbonyl group .The 1H NMR spectra of 4 in (DMSO-d6) showed a triplet at δ 1.18 ppm, quartet at δ 4.12 ppm corresponds to CH2CH3 group and singlet at δ 4.04 ppm corresponds to CH2 protons. In addition, mass spectrum of 4 showed a molecular ion peak at m/z 464 corresponding to a molecular formula C25H21ClN2O3S (Scheme 1).Reaction 1 with phenacyl bromide in the presence of catalytic amount of triethylamine (TEA) in ethanol [25] afforded the corresponding 2-(benzoylmethylthio)-5,5- di-phenyl-imidazolidin-4-one 5. Compound 5was used as a key for synthesis of different heterocyclic compounds via treatment with different reagents. Thus, reaction of imidazolidin-4-one 5 derivative with benzaldehyde in the presence of piperidine [26] to yield the arylidine derivatives 6. 1H-NMR spectrum of 6 in (DMSO-d6) show the disappearance of CH2 protons observed in starting 5 at δ 4.10 ppm and the appearance multiplet signals at δ 7.09-7.84 ppm corresponding to the aromatic protons together with C=CH proton.On the other hand, reaction of compound 5 with carbon disulphide in basic dimethylformamide (DMF) gave non- isolated potassium monothiolate derivatives 7. Alkylation of compound 7 by using methyl iodide and ethyl chloroacetate [27] give methyl dithioate derivative 8 and ethyl (3-phenyl- propanedithioyl)acetate derivative 9.Structures 8 and 9 were established by the correct analyses and spectroscopic data (see Experimental Section).Treatment of compound 5 with phenyl isothiocyanate in basic DMF give non-isolated potassium salt 10 and then adduct ethyl chloroacetate [28] cycloalkylation occurred to afford the 1,3-thiazolidin-4-one derivative 11. The formation of thiazoldin-4-one 11 may be rationalized through the first S-alkylation, then cyclisation to give 11.The structure of the isolated product 11 was elucidated on the basis of their spectral data. The IR spectrum of exhibits bands at 1728 cm-1 to thiazolidinone CO. The 1H-NMR spectra revealed a thiazolidinone CH2 signal at δ 4.04.Furthermore, compound 5 undergoes Gewald reaction via treatment with elemental sulfur and 2-(1H-benzimidazol- 2-yl) acetonitrile in absolute ethanol and DMF (2:1) containing a catalytic amount of morpholine [29] afforded the corresponding 2-[3-phenyl-thiophen-2-ylthio]-5,5- diphenyl- imidazolidin-4-one derivative 12 (Scheme 1). Structure of 12 was confirmed on the basis of analytical and spectral data. The IR spectra showed the disappearance of CN group and showed an absorption bands at 3245, 3145, 3130 cm-1 corresponding to the NH2, NH groups .1H-NMR spectra showed NH2 and NH protons appeared at δ 4.81 and δ13.03 ppm respectively as s singlet signals. Mechanism for the formation of 12 in which the intermediate I is obtained first, then cyclization to give 12 (Figure 1). Several alkylated derivatives were obtained from 1-(4-substituted phenyl)- 4,5-diphenyl-1H-imidazole- 2-thione 2a,b. Thus, upon treatment of 2a,b with benzyl chloride, chloroacetonitrile, chloroacetone, phenacyl bromide and chloroacetamide in the presence of catalytic amount of TEA in ethanol [30] afforded the corresponding S-alkylated derivatives 13 a-d and 15a-e respectively (Scheme 2). The structures of new compounds were supported on the basis of elemental analyses and spectral data. For example, IR spectra of compounds 13c,d revealed absorption bands for CN at 2246,2240 cm−1 and characteristic absorption band at 1711,1680,1691,1683,1676 cm−1 for the C=O confirming the structure of 15 a-e.
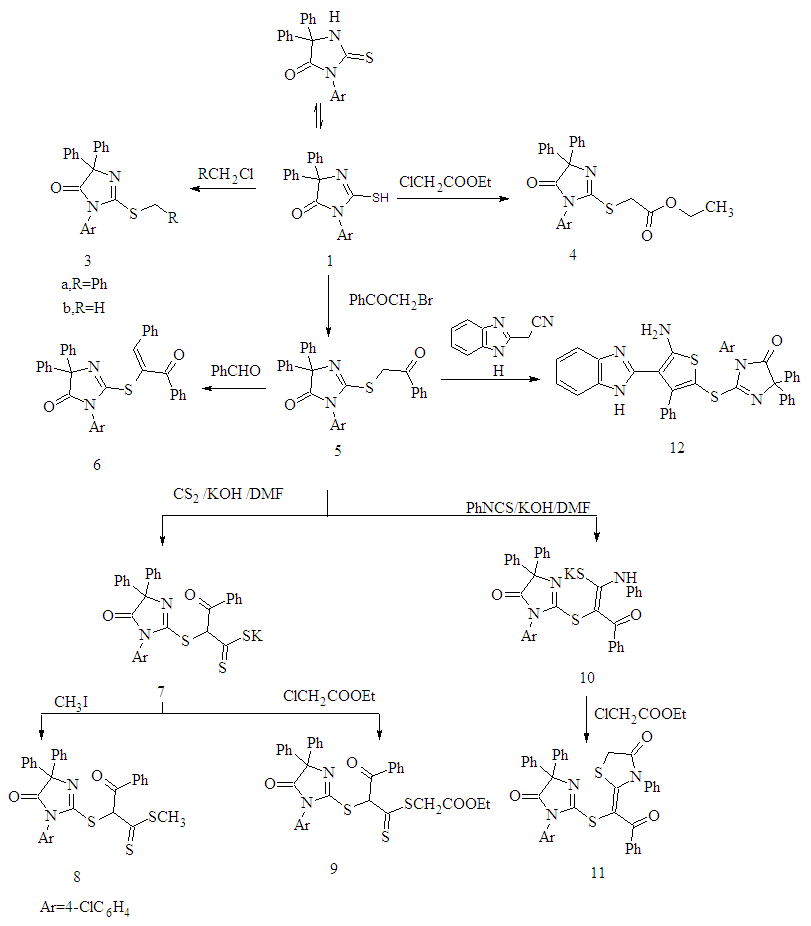 | Scheme 1. Synthesis of compounds 3-12 |
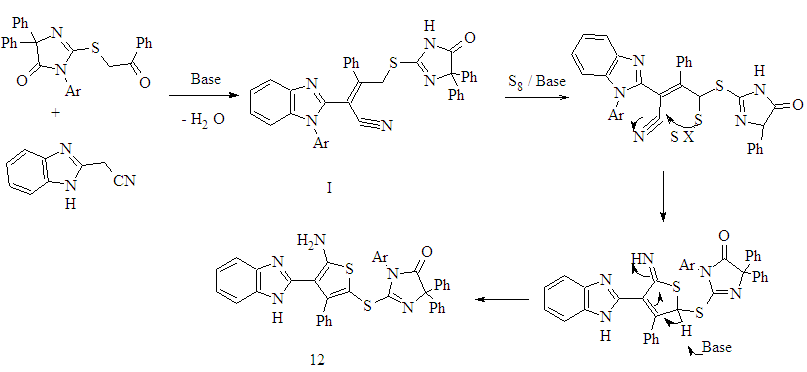 | Figure 1. The proposed mechanism formation 2(3- phenyl -thiophen-2-ylthio]-imidazolidin-4-one 12 |
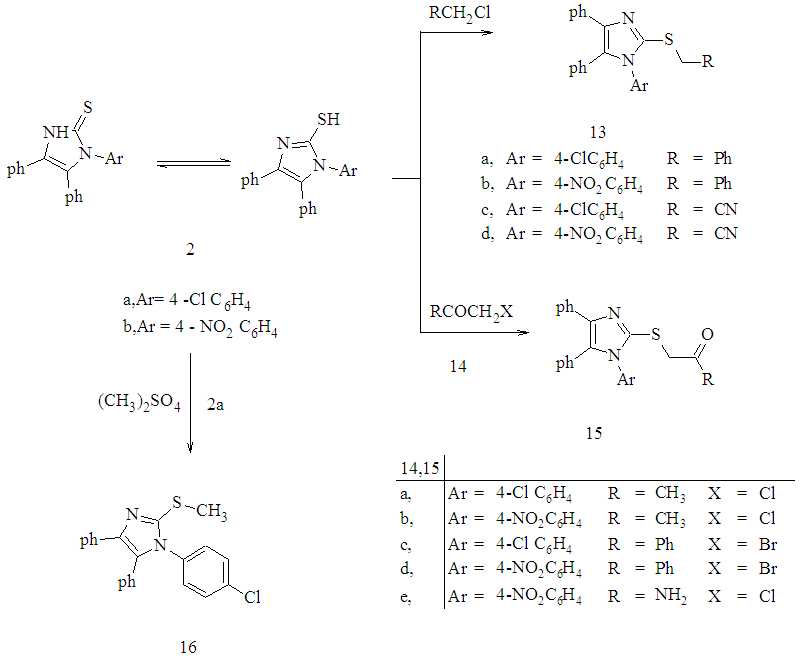 | Scheme 2. Synthesis of Compounds 13-16 |
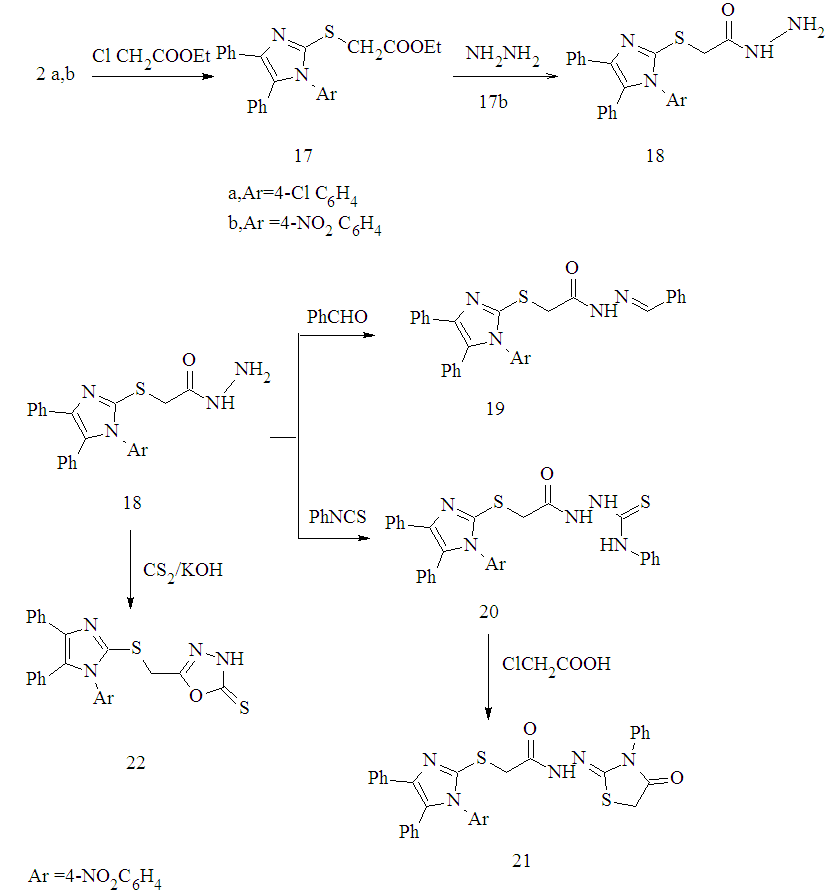 | Scheme 3. Synthesis of Compounds 17-22 |
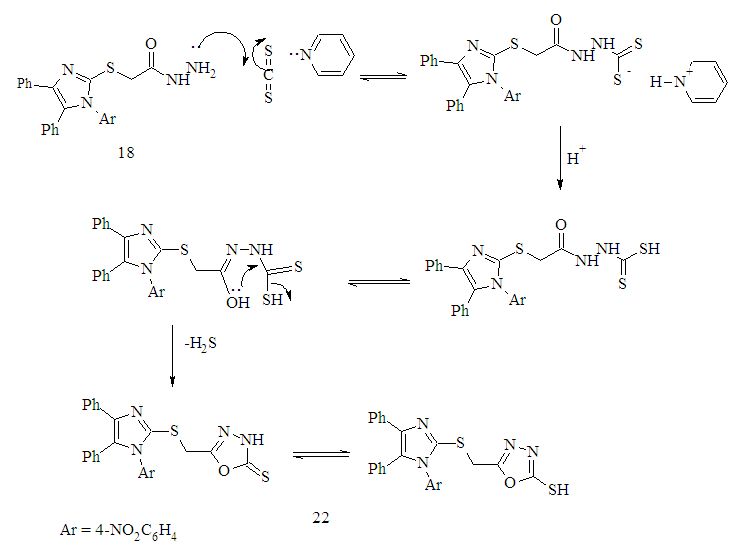 | Figure 2. The proposed mechanism formation 1,3,4-oxadiazole-2-thione derivative 22 |
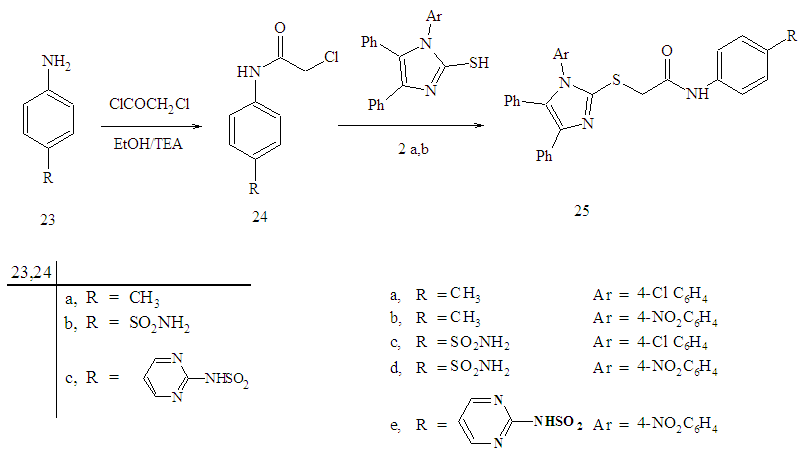 | Scheme 4. Synthesis of compounds 24a-c and 25-e |
3.2. Biological Activity
- 3.2.1. Antimicrobial ActivitySynthesized 4, 5, 11, 13a, 15e, 17a, 25c and 25e were evaluated for antibacterial and antifungal activityAntibacterial Activity Synthesized compounds 4,5,11,13a,15e,17a,25c and 25e were screened for their antibacterial acti- vities in vitro against Gram-positive Staphylococcus aureus (RCMB 0100010) and Bacillus subtilis (RCMB 010067) and Gram-negative Pseudomonas aeuroginosa (RCMB010043) and Escherichia coli (RCMB 010052) Ampicillin and gentamicin were used as references to evaluate the potency of the tested compounds. The inhibitory effects of the synthetic compounds against these organisms are given in Table 1, Figure 3.In general, most of the tested compounds revealed better activity against the Gram-positive rather than the Gram- negative bacteria. All test compounds were found to be inactive against Pseudomonas aeuroginosa (RCMB 010043). Compounds 17a 25c and 25e exhibited excellent activity against Staphylococcus aureus (RCMB 0100010) and Bacillus subtilis (RCMB 010067) compared with the standards ampicillin.While compounds 4,5,11, 13a and 15e showed moderate to weak activity against Staphylococcus aureus (RCMB 0100010) and Bacillus subtilis (RCMB 010067) compared with the standards ampicillin. On other hand, compounds 17a and 25e strong activities against Escherichia coli (RCMB 010052) compared with the standards gentamicin. While compounds 4, 5, 11, 13a, 15e and 25c moderate to week activity against Escherichia coli (RCMB 010052 ) compared with the standards gentamicin.Antifungal ActivityThe newly synthesized compounds 4, 5, 11, 13a, 15e, 17a, 25c and 25e were screened for their antifungal activities in vitro against Aspergillus fumigatus (RCMB02568), Syncephalastrum racemosum (RCMB05922), Geotrichum candidum (RCMB05097) and Candida albicans (RCMB 05036). The inhibitory effects of the synthetic compounds against these organisms are given in Table 2, Figure 4.Compound 11, 25c and, 25e showed strong activities against Aspergillus fumigatus (RCMB02568) , Syncephalastrum racemosum (RCMB05922) and Geotrichum candidum (RCMB05097) comparable to Amphotericin B. On the other hand compounds 4, 5 13a, 15e, and 17a showed moderate to weak activities against Aspergillus fumigatus (RCMB02568), Syncephalastrum racemosum (RCMB05922) and Geotrichum candidum (RCMB05097) comparable to Amphotericin B .Furthermore, all test compound were found to be inactive against Candida albicans (RCMB 05036)In vitro Antioxidant Activity-DPPH radical methodIn general the principle of DPPH method is based on nitrogen centered stable free radical DPPH has often been used to characterize antioxidants. It is reversibly reduced and the odd electron in the DPPH free radical gives a strong absorption maximum at 517 nm, which is purple in color. This property makes it suitable for spectrophotometric studies. A radical scavenging antioxidant reacts with DPPH stable free radical and converts into 1,1-diphenyl-2- picrylhydrazine (non radical). The change in the absorbance produced in this reaction has been used to measure antioxidant property. [42-43]. The free radical-scavenging activity of the synthesized molecules was measured in terms of hydrogen donating or radical scavenging ability using the stable radical DPPH. The reduction capacity of DPPH radical which is induced by antioxidant was determine by the decrease in its absorbance at 517nm. It is visually noticeable as a change in colour from purple to yellow. This was expressed as the inhibition percentage.The antioxidant activity of the newly synthesized compounds 1,2,3b,4,5,13c,15a,15c, 16 and 20 were evaluated by DPPH method. The results are shown in Table 3 and figure 5 and 6. The data in Table 3 showed clearly that compounds 2 and 20 have high activity while compound 11,3b and 5 showed weak activities. On the other hand, compounds 4,13c,15a,15c and 16 showed no activities.
|
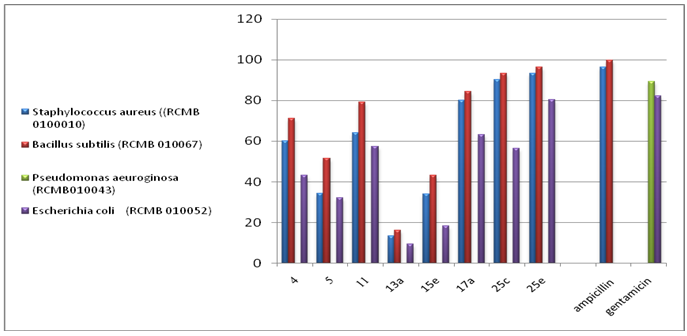 | Figure 3. Graphical Representation of Antibacterial Activity of Some Synthesis Compounds |
|
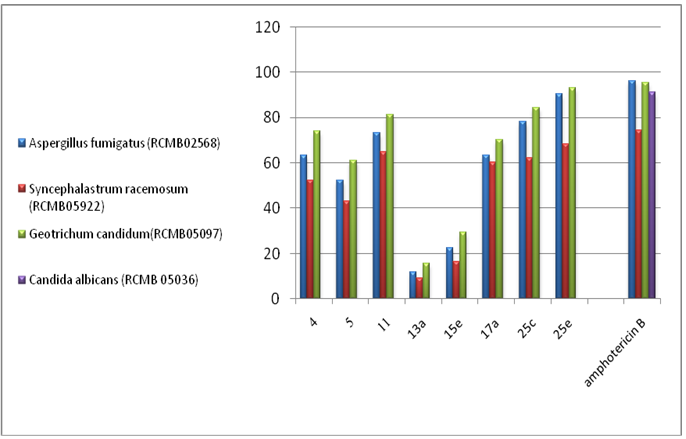 | Figure 4. Graphical Representation of Antifungal Activity of Some Synthesis Compounds |
|
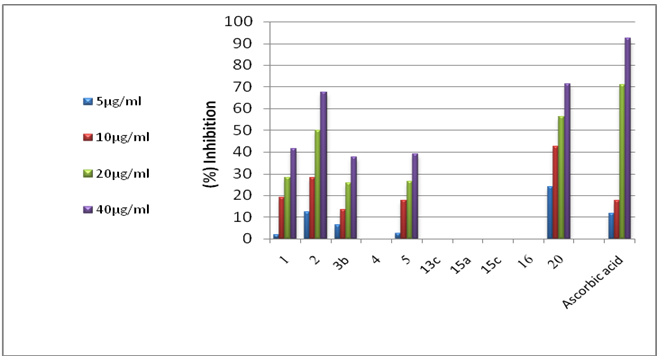 | Figure 5. Radical Scavenging Potential of The Synthesis Compounds By DPPH Method at Different Concentration |
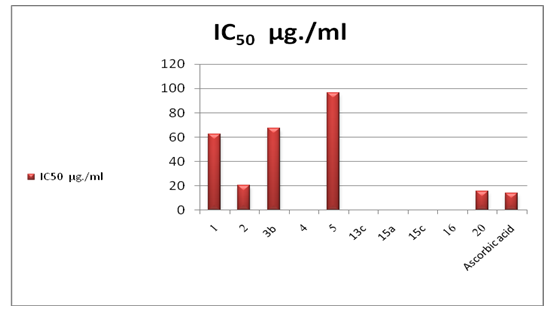 | Figure 6. IC50 Value of The Newly Synthesized Compounds |
4. Conclusions
- Novel series of 2-thio-substituted- imidazoles and imidazolidine were synthesis. Screening for some selected compounds was carried for their potential antibacterial, antifungal and antioxidant activity using DPPH free radical scavenging method. Compound 25e exhibited excellent activity against Staphylococcus aureus, Bacillus subtilis and Escherichia coli compared with the standards drugs. 25e showed strong activities against Aspergillus fumigatus, Geotrichum candidum comparable to Amphotericin B. compound 2 and 20 showed good DPPH scavenging activity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML