| [1] | Moloney, M. G. Nat. Prod. Rep.2002, 19, 597-616. DOI: 10.1039/B103777N, Review Article |
| [2] | Tandon, V. K.; Yadav, D. B.; Maurya, H. K.; Chaturvedi, A. K.; Shukla, P. K. Med. Chem. 2006, 14, 6120-6126. DOI: 10.1016/j.bmc.2006.04.029 |
| [3] | Kotharkar, S. A.; Shinde, D.B. Bioorg. Med. Chem. Lett. 2006, 16, 6181-6184. |
| [4] | Mashevskaya, I. V.; Makhmudov, R. R.; Aleksandrova, G. A.; Golovnira, O. V.; Duvalov, A. V.; Maslivets. A. N. Pharm. Chem. J. 2001, 35,196 -198. |
| [5] | Vyas, D. A.; Chauhan, N. A.; Parikh, A. R. Indian J. Chem. 2007, 46B, 1699-1702. |
| [6] | Carta, A.; Loriga, M.; Paglietti, G.; et al. Eur. J. Med. Chem. 2004, 39,195- 203. |
| [7] | Seitz, L. E.; Suling, W. J.; Reynolds, R. C. J. Med. Chem. 2002, 45,5604-5606. |
| [8] | Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Bioorg. Med. Chem. 2003, 11, 2149-2156. |
| [9] | Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. J. Med. Chem. 2005, 48, 2019-2025. |
| [10] | Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Eur. J. Med. Chem. 2003, 38,791-800. |
| [11] | Burguete, A. ; Pontiki, E.; Litina, D. H.; et al. Bioorg. Med. Chem. Lett. 2007, 17, 6439-6443. |
| [12] | Wagle, S.; Adhikari, A. V.; Kumari, N. S. Indian J. Chem. 2008, 47B, 439-448. |
| [13] | Ishikawa, H.; Sugiyama, T.; Kurita, K.; Yokoyama, A. Bioorg.Chem.2012, 41-42, 1-5. |
| [14] | Loriga, M.; Piras, S.; Sanna, P.; Paglietti, G. Farmaco 1997, 52(3), 157-166. |
| [15] | Crowther, A. F.; Curd, F.H.S.; Davey, D.G.; Stacey, G.J. J. Chem. Soc. 1949, 1260-1262. |
| [16] | Rangisetty, J.B.; Gupta, C.N.V.H.B.; Prasad, A,L. Srinivas, P.; Sridhar, N.; Parimoo, P.; Veeranjaneyulu, A. J. Pharmacy, Pharmacology 2001, 53, 1409 -1413. |
| [17] | Sarges, R.; Howard, H.R.; Browne, R.G.; Lebel, L.A.; Seymour, P.A.; Koe, B. k. J. Med. Chem. 1990, 33, 2240 -2254. |
| [18] | (a) Jian, F. F.; Zhao, P.S. J. Mol. Struct. 2004,705, 133-139. (b) Cheon, H. G.; Lee, C. M.; Kimb, B.T.; Hwangb, K. J. Bioorg. Med. Chem. Lett 2004, 14, 2661. (c) Kaupp, G.; Naimi-Jamal, M. R. Eur. J. Org. Chem. 2002, 8, 1368-1373. (d) Wang, L.; Liu, J.; Tian, H.; Qian, C.Synth. Commun. 2004, 34, 1349-1358. |
| [19] | Monge, A.; Palop, J.A.; Urbasos, I.; Fernández-Alvarez, E. J. Heterocyclic Chem.1989, 26(6), 1623-1626. DOI: 10.1002/jhet.5570260621 |
| [20] | Kurusawa, Y.; Muramatsu, M.; Okamoto, Y.; Takada, A. Heterocycl. Chem. 1986, 23, 637. |
| [21] | Phadke, R. C.; Rangnekar, D.W. Bull. Chem. Soc. Japan 1986, 59, 1245-1247. |
| [22] | Ramli, Y.; Moussaif, A.; Karrouchi, K; Essassi, E. J. Chem.2014, 2014, 1-21. |
| [23] | Dell, A.; Williams, D. H.; Morris, H. R. J. Am. Chem. Soc. 1975, 97, 2497- 2502. |
| [24] | Dirlam, J. P.; Presslitz, J. E. J. Med. Chem. 1978, 21, 483-485. |
| [25] | Dirlam, J. P.; Czuba, L. J.; Dominy, B.W.; et al. J. Med. Chem. 1979, 22, 1118-1121. |
| [26] | Monge, A.; Martinez-Crespo, F. J.; De Cerain, A. L.; et al. J. Med. Chem.1995, 38, 4488-4494. |
| [27] | Monge, A.; Palop, J. A.; De Cerain, A. L.; et al. J. Med. Chem. 1995, 38, 1786-1792. |
| [28] | Montoya, M. E.; Sainz, Y.; Ortega, M.A.; De Cerain, A. L.; Monge, A. Farmaco 1998, 53, 570-573. |
| [29] | Ortega, M. A. ; Sainz, Y.; Montoya, M. E. ; De Cerain, A. L. ; Monge, A. Pharmazie 1999, 54, 24-25. |
| [30] | Sainz, Y. ; Montoya, M. E. ; Martinez-Crespo, F. J.; Ortega, M. A. ; de Cerain, A. L.; Monge, A. Arzneim.-Forsch. 1999, 49, 55-59. |
| [31] | Ortega, M. A.; Morancho, M. J.; Martinez-Crespo, F.J.; Sainz,Y.; Montoya, M. E.; de Cerain, A.L.; Monge, A. Eur. J. Med. Chem. 2000, 35, 21-30. |
| [32] | Ortega, M. A.; Montoya, M. E.; Jaso, A.; Zarranz, B.; et al. Pharmazie 2001, 56, 205-207. |
| [33] | Carta, A.; Paglietti, G.; Rahbar, N.M.E.; et al. Eur. J. Med Chem. 2002, 37, 355-366. |
| [34] | Ortega, M. A.; Sainz, Y.; Montoya, M. E.; et al. Arzneim.-Forsch. 2002, 52, 113-119. |
| [35] | Aldana, I.; Ortega, M. A.; Jaso, A.; et al. Pharmazie 2003, 58, 68-69. |
| [36] | Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Bioorg. Med. Chem. 2003, 11, 2149-2156. |
| [37] | Aguirre, G.; Cerecetto, H.; Di Maio, R.; et al. Bioorg. Med. Chem Lett. 2004, 14, 3835-3839. |
| [38] | Kim, Y. B.;Kim, Y. H.; Park, J. Y.; Kim, S. K. Bioorg. Med. Chem. Lett. 2004, 14, 541-544. |
| [39] | Perez-Melero, C.; Maya, A. B.; del Rey, B.; et al. Bioorg. Med. Chem. Lett. 2004, 14, 3771-3774. |
| [40] | Singh, S. K.; Saibaba, V.; Ravikumar, V. Bioorg. Med. Chem. 2004, 12, 1881-1893. |
| [41] | Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A.; et al. Bioorg. Med. Chem. 2004, 12, 3711-3721. |
| [42] | Carta, A.; Corona, P.; Loriga, M. Curr. Med. Chem. 2005, 12, 2259-2272. |
| [43] | Lima, L. M. ; Zarranz, B.; Marin, A. J. Heterocycl. Chem. 2005, 42,1381-1385. |
| [44] | Zarranz, B.; Jaso, A.; Aldana, I.; et al. Arzneim.- Forsch. 2005, 55,754-761. |
| [45] | Urquiola, C.;Vieites, M.; Aguirre, G. ;et al. Bioorg. Med. Chem. 2006, 14, 5503-5509. |
| [46] | Zarranz, B.; Jaso, A.; Lima, L. M. Braz. J. Pharm. Sci. 2006, 42, 357-361. |
| [47] | Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; et al. Bioorg. Med. Chem. Lett. 2007, 17, 6439-6443. |
| [48] | Solano, B.; Junnotula, V.; Marín, A.; et al. J. Med. Chem. 2007, 50, 5485-5492. |
| [49] | Marín, A. ; Lima, L. M. ; Solano, B. ; et al. Exp. Parasitol. 2008,118, 25-31. |
| [50] | Vicente, E.; Charnaud, S.; Bongard, E. ; et al. Molecules 2008, 13, 69-77. |
| [51] | Vicente, E.; Lima, L. M.; Bongard, E. Eur. J. Med. Chem. 2008, In Press. doi:10.1016/j.ejmech.2007.1011.1024. |
| [52] | Francis, J.; Landquist, J.K.; levi, A.A.; Silk, J. A. ;Thorpe, J.M. J. Biochem. 1956, 63(3), 455-457. |
| [53] | Dirlam, J.P.; Presslitz, J.E.;Williams, B.J. J. Med. Chem. 1983, 26(8),1122-1126. |
| [54] | Vicente, E.; Villar, R.; Burguete, A.; et al. Antimicrob. Agents Chemother 2008, 52, 3321-3326. |
| [55] | Hanan, R. M.; Ashraf, M. A.; Omneya, K. M. Arch Pharm Res.2004, 11, 1093-1098. |
| [56] | Asha, B.; Abdul, B. R.; Amir, A. Eur. J. Med. Chem. 2009, 44,1317-1325. |
| [57] | Jin-Hwa, C.; Ok, J.J.;Mi, C. J.; et al. Bioorg. Med. Chem. Lett. 2005, 15, 3380-3384. |
| [58] | Yang, Y.; Zhang, S.; Wu B, et al. Chem.Med.Chem. 2012, 7(5), 823-835. Sastry, R.; Marwah, C.V.; Marwah, P.; A K.; Rao, G.S. Indian. J. Chem.1989, 28B, 885-891. |
| [59] | Li, J.J. J.Org. Chem. 1999, 64, 8425-8427. |
| [60] | Patel, N.; Bergman, J.; Graslund, A. Eur.J.Biochem.1991, 197, 597-604. |
| [61] | Miyagi, T.; Yamamoto, H. Japanese Patent 1967, 17747.Chem. Abstr. 1968, 69, 10475. |
| [62] | Cole, S.P.; Bhardwaj, G.; Gerlach, J.H.; et al. Science 1992, 258, 1650-1654. |
| [63] | Lawrence, D. S.; Copper, J. E.; Smith, C.D. J. Med. Chem. 2001, 44(4), 594-601. |
| [64] | Yoo, H.W.; Suh, M.E.; Park, S.W. J. Med. Chem. 1998, 41,4716 - 4722. |
| [65] | Weng, Q.; Wang, D.; Guo, P.; et al. Eur. J. Pharmacology 2008, 581(3), 262-269. |
| [66] | Fedora, G.; Francesca, A.; Osvaldo, G. D.; Antonella, B.; Antonio, G.; Nouri, N. Bioorg. Med.Chem.2007, 15, 288-294. |
| [67] | Heesoon, L.; Sungmoon, C.; Kwon, N.; Jae-Kyung, J.K.; Jung, C. ;Sung, Y. I. Bioorg. Med. Chem 2004, 14, 1235-1237. |
| [68] | Ries, U. J.; Priepke, H. W. M.; Hauel, N. H. Bioorg. Med. Chem. Lett. 2003, 13(14), 2297-2302. |
| [69] | Balzarini, J.; Karlsson, A.; Meichsner, C. J. Virol 1994, 68(12), 7986-7992. |
| [70] | Balzarini, J.; Pelemans, H. ; Riess, G. J. infect Dis 1997, 176(5), 1392-1397. |
| [71] | Balzarini, J.; De clereg, E. ; Carbonez, A. AIDS Res Hum Retroviruses 2000, 16(6), 517-528. |
| [72] | Abu-Hashem, A. A.; Gouda, M.A.; Badria, F. A. Eur. J. Med. Chem.2010, 45, 1976-1981. DOI:10.1016/j.ejmech.2010. 01.042 |
| [73] | Kumar A, kumar S, Saxena A, Mozumdar S. Catal. Commun. 2008, 9,778-784. |
| [74] | Heravi, M. M.; Taheri, S.; Bakhtiari, K.; Oskooie, H. Catal.Commun.2007, 8, 211-214. |
| [75] | Heravi, M. M.; Taheri, S.; Bakhtiari, K.; Oskooie, H.; Javadi, N.M. Arkivoc 2006, 16, 16-22. |
| [76] | Kotharkar, S.A.; Shinde, D.B. J .Iranian Chem. Soc. 2006, 3, 267-271. |
| [77] | Ajaikumar, S.; Pandurangan, A. Applied Catalysis A: General 2009, 357,184-192. |
| [78] | Bandyopadhyay, D.; Mukherjee, S.; Rodriguez, R. R.; Banik, B. K. Molecules 2010, 15, 4207-4212. |
| [79] | Wallace, J.M.; Bjorn, C.G.; Tamariz, J.; Akhmedov, N. G.; Hurley, M.T. Tetrahedron 2008, 64, 9675-9684. |
| [80] | Rao, V. J.; Palaniappan, S. J. Mol. Catal. 2007, 265, 227-230. |
| [81] | Lin, P.-Y.; Hou, R.-S.; Wang, H.-M.; Kang, I.-J.; Chen, L.-C. J. Chin Chem. Soc. 2009, 56, 683-687. |
| [82] | Darabi, H. R.; Mohandessi, S.; Aghapoor, K.; Mohasenzadeh, F.A. Catal. Commun. 2007, 8, 389-392. |
| [83] | Sadjadi, S.; Hekmatshoar, R.; Ahmadi, S. J.; Hosseinpour, M.; Outokesh, M. Synth Commun. 2010, 40, 607- 614. |
| [84] | Meshram, G. A.; Deshpande, S. S. Eur. J. Chem. 2013, 4(4), 422‐424.; Katkar, S. S.; Mohite, P. H.; Gadekar, L. S.; Arbad, B. R.; Lande, M. K. Cen. Eur. J. Chem. 2010,8,320. |
| [85] | Das, B.; Venkateswarlu, K.; Suneel, K.; Majhi, A. Tetrahedron Lett. 2007, 48, 5371-5374. |
| [86] | Kumar, A. V.; Reddy, V. P.; Rao, K. R. Synlett 2010, 17, 2571-2574. |
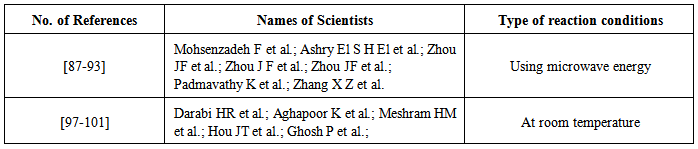
| [87] | Mohsenzadeh, F.; Aghapoor, K.; Darabi, H. R. J. Braz. Chem. Soc. 2007, 18, 297-303. |
| [88] | El- Ashry, S. H.; Atta, K. F.; Aboul-Ela, S.; Beldi, R. J. Carbohydrate Chem. 2007, 26, 1-16. |
| [89] | Zhou, J.-F.; Gong, G.-X.; An, L.-T.; Liu, Y.; Zhu, F.-X.; Zhu, Y.-L.; Ji, S. J. Synlett 2008, 20, 3163-3166. |
| [90] | Zhou, J.F.; Gong, G. X.; Shi, K.B.; Zhi, S. J. Chinese Chem. Lett. 2009, 20, 672-675. |
| [91] | Zhou, J.-F.; Gong, G.-X.; Zhi, S.-J.; Duan, X.-L. Synth Commun. 2009, 39, 3743. |
| [92] | Padmavathy, K.; Nagendrappa, G.; Geetha, K.V. Tetrahedron Lett. 2011, 52, 544-547. |
| [93] | Zhang, X.-Z.; Wang, J.-X.; Bai, L. Synth Commun. 2011, 41, 2053-2063. |
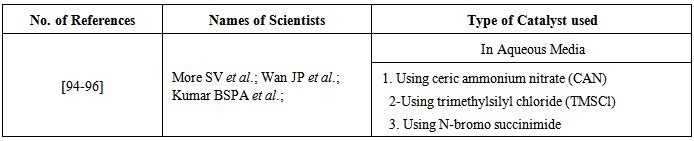
| [94] | More, S. V.; Sastry, M. N.V.; Yao, C.-F. Green Chem. 2006, 8, 91-95. |
| [95] | Wan, J.-P.; Gan, S.-F.; Wu, J.-M.; Pan, Y. Green Chem. 2009, 11, 1633-1637. |
| [96] | Kumar, B. S. P. A.; Madhav, B.; Reddy, K. H. V.; Nageswar, Y.V.D. Tetrahedron Lett. 2011, 52, 2862-2865. |
| [97] | Darabi, H. R.; Tahoori, F.; Aghapoor, K.; Taala, F.; Mohsenzadeh, F. J. Braz. Chem. 2008, 19, 1646-1652. |
| [98] | Aghapoor, K.; Darabi, H. R.; Mohsenzadeh, F.; Balavar, Y.; Daneshyar, H. Transition Met. Chem. 2010, 35, 49-53. |
| [99] | Meshram, H. M.; Kumar, G. S.; Ramesh, P.; Reddy, B. C. Tetrahedron Lett. 2010, 51, 2580-2585. |
| [100] | Hou, J.-T.; Liu, Y.-H.; Zhang, Z.-H. J. Heterocyclic Chem. 2010, 47, 703-706. |
| [101] | Ghosh, P.; Mandal, A. Advances Applied Sci. Res. 2011, 2, 255-260. |
| [102] | Chandrasekhar, S.; Reddy, N. Tetrahedron Letters 2010, 51, 3623-3625. |
| [103] | Dong, F.; Kai, G.; Zhenghao, F.; Xinli, Z.; Zuliang, L. Catal. Commun.2008, 9, 317-320. |
| [104] | Antoniottia, S.; Duach, E. Tetrahedron Letters 2002, 43, 3971-3973. |
| [105] | Jeffery, M. W.; Bjo¨rn, C.G.; et al. Tetrahedron 2008, 64, 9675-9684. |
| [106] | Raj, K.B. Heterocyclic chemistry .4th ed. New Delhi: New Age International publishers 2005, 535-538. |
| [107] | Zmujdzin, l. b. A. Pol. Patent 1974, 69644. Chem. Abstr. 1974, 81, 77966. |
| [108] | Noorulla, S.; Sreenivasulu, N. Inter. J. Res. Pharm. Biomed. Sci. 2011, 2 (3), 1100-1106. |
| [109] | Shabaan, M.; Taher, T. A.; Osman, O. E. Eur. J. Chem. 2011, 2 (3), 365‐371. |
| [110] | Gorbunova, E. A.; Mamedov, V. A. Russian J. Organic Chem. 2006, 42(10), 1528-1531. |
| [111] | Islami, R. M. ; Hassani, Z. Arkivoc 2008, (xv), 280-287. |
| [112] | Taylor, E. C.; Thompson, M. J. J. Org. Chem.1961, 26, 3511. DOI: 10.1021/jo01067a604 |
| [113] | Kumar, N.; Sharma, P.; Kaur, N.; Pareek, A. J. Applicable Chem. 2013, 2 (2), 143-149. |
| [114] | Atkinson, C. M.; Brown, C. W.; Simpson, J. C. E. J. Chem. Soc. 1956, 26-30. |
| [115] | Bandyopadhyay, D.; Mukherjee, S.; Rodriguez, R. R.; Banik, B. K. Molecules 2010, 15, 4207-4212. |
| [116] | Kleineweischede, A.; Mattay J. Eur. J. Org. Chem. 2006, 4, 947-957. DOI: 10.1002/ejoc.200500548 |
| [117] | Sherman, D.; Kawakami, J.; He, H.Y.; Dhun, F.; Weitao, P.; Labelle, X. Y. M. Tetrahedron Lett.2007, 48, 8943-8946. |
| [118] | Thakuria, H.; Das, G. J. Chem. Sci. 2006, 118(5), 425-428. |
| [119] | Gris, J.; Glisoni, R.; Fabian, L.; Fernandez, B.; Moglioni, A.G. Tetrahedron Lett. 2008, 49, 1053-1056. |
| [120] | Rostamizadeh, S.; Jafari, S. Indian. J. Heterocyclic Chem. 2001, 10, 303-304. |
| [121] | Ratnadeep, V.; Ghadage; Pramod, J. S. J. Chem. Pharm. Res. 2011, 3(5), 260-266. |
| [122] | Khan, S.A.; Mullick, P.; Pandit, S.; Kaushik, D. Acta Poloniae Pharmaceutica - Drug Research 2009, 66(2), 169-172. |
| [123] | Mahmoud, A. A.; Mohamed, M.Y. Der Pharma Chemica 2012, 4 (3), 1323-1329. |
| [124] | Badran, M. M.; Moneer, A. A.; Refaat, M. H.; El-Malah, A. A. J. Chinese Chemical Society 2007, 54, 469-478. |
| [125] | Heravi, M.M.; Bakhtiari, K.; Tehrani, M.H.; Javadi,N. M.; Oskooie, H.A. Arkivoc 2006, xvi, 16-22. |
| [126] | Lin, S.K. Molecules 1996, 1, 37-40. |
| [127] | Ruiz, M. D.; Autino, C. J.; Quaranta, N.; V´azquez, G. P.; Romanelli, P. G. The Scientific World J. 2012, 2012, Article ID 174784, 8.doi:10.1100/2012/174784. |
| [128] | Cai, J.J.; Zou, J.P.; Pan, X.Q.; Zhang, W. Tetrahedron Lett. 2008, 49, 7386-7390. |
| [129] | Heravi, M. M.; Baghernejad, B.; Oskooie, H. A. Tetrahedron Lett. 2009, 50,767-769. |
| [130] | Bhosale, R.S.; Sarda, S.R.; Ardhapure, S.S; et al. Tetrahedron Lett. 2005, 46, 6345- 6348. |
| [131] | Khaksar, S.; Rostamnezhad, F. Bull. Korean Chem. Soc. 2012, 33, 2581-2584. |
| [132] | Brown, D.J. The Chemistry of Heterocyclic Compounds, Quinoxalines: Supplement II, John Wiley & Sons, INC., New York 2004, 1-92. |
| [133] | Iveta, W. Acta Universitatis Palackianae Olomucensis Facultas Rerum Naturalium 2002, 46, 771. |
| [134] | Ali, M.M. Molecules 2000, 5, 864-873. |
| [135] | Norma, F.; Santos-Sánchez; Salas-Coronado, R. Arkivoc 2008, v, 187-199. |
| [136] | Varano, F. Eur. J. Med. Chem. 2001, 36, 203-209. |
| [137] | Ohle, H.; Heilscher, M. Ber. Dtsch. Chem. Gas 1941, 74B, 13-19. |
| [138] | Henseke, G.; Lemke, W. Chem. Ber. 1958, 91, 101. |
| [139] | Yan, L.; Liu, F.; Dai, G.; Liu, H. Bioorg. Med. Chem. Lett. 2007, 17, 609-612. |
| [140] | Leese, C. L.; Rydon, H.N. J.Chem.Soc. 1955,303-309. |
| [141] | El Khadem, H. Advances in Carbohydrate Chemistry 1965, 20, 139-181. doi:10.1016/S0096-5332(08)60298-2. |
| [142] | Bar1trop, J.A.; Richards, C.G.;Russell, D.M. J.Chern.Soc. 1959, 1423. |
| [143] | Schellenberg, M. He1v.Chirn. Acta. 1970, 53, 1151. |
| [144] | Mostafa, A.M.; Aboulela, L.S.; Sallam, E. A. M.; Louis, F. F.; Anthonsen, T. Green and Sustainable Chem. 2012, 2, 71-75. |
| [145] | Toman, J.; Klicnar, J.; Macháček, V. Collect. Czech. Chem. Commun. 1978, 43, 2179-2189. Doi:10.1135/cccc19782179. |
| [146] | Wu, X.H.; Liu, G.; Zhang, J. Mol Divers 2004, 8(2),165- 174. |
| [147] | Gao, X.M.; Luo, W.P.; Shen, X.F. Pesticides 1998, 37(7), 12-13. |
| [148] | Carta, A.; Sanna, P.; Gherardini, L. Il Farmaco, 2001, 56, 933 -938. |
| [149] | Bajpai, S.; Singh, S.; srivastava, V. Inter. J. Innovative Res. Sci., Engineering and Technology 2013, 2(9), 2319-8753. |
| [150] | Sherman, D.; Kawakami, J.; Hai-Ying, H.; et al. Tetrahedron Lett. 2007, 48, 8943-8946. |
| [151] | Xie, F.; Zhang, M.; Jiang, H.; Chen, M.; Lv, W.; Zheng, A.; Jian, X. Green Chem. 2014, First published online 21 Aug 2014. DOI: 10.1039/C4GC01316F. |
| [152] | Ghadari, R.; Hajishaabanha, F.; Aghaei, M.; Shaabani, A.; Weng, N.S. Mol Divers 2012, 16, 453-461. |
| [153] | Surikova, O.V.; Aliev, Z. G.; Polygalova, N. N.; Mikhailovskii, A. G.; Vakhrin, M I. Russian J. Organic Chem. 2008, 44, 901-905. |
| [154] | Karami, B.; Khodabakhshi, S. J. Serb. Chem. Soc.2011, 76 (9), 1191-1198. |
| [155] | Karami, B.; Damavandi, J. A.; Bayat, M.; Montazerozohori, M. J. Serb. Chem. Soc. 2006, 71: 27. |
| [156] | Karami, B.; Montazerozohori, M.; Habibi, M. H. Bull. Korean Chem. Soc. 2005, 26, 1125-1128. |
| [157] | Karami, B.; Montazerozohori, M. ; Karimipour, G.; Habibi, M. H. Bull. Korean Chem. Soc. 2005, 26, 1431. |
| [158] | Karami, B.; Montazerozohori, M. ; Habibi, M. H. ; Zolfigol, M. A. Heterocyclic Commun. 2005, 11, 513. |
| [159] | Karami, B.; Montazerozohori, M.; Habibi, M. H. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2825-2831. |
| [160] | Montazerozohori, M.; Karami, B. Helv. Chim. Acta 2006, 89, 2922- 2926. |
| [161] | Montazerozohori, M.; Karami, B.; Aziz, M. Arkivoc 2007, (i), 99-104. |
| [162] | 162.Karami, B.; Montazerozohori, M. Molecules 2006, 11, 720-725. |
| [163] | Isikdag, İ.; Özkay, Y.; İncesu, Z. Turk J. Pharm. Sci. 2011, 8 (2), 179-188. |
| [164] | Berezoviskii, V. M.; Polyakova, N. A.; Tulchinskaya, L. S.; Geterotsikl, K. Chem. Abstr. 1968, 68, 78254. |
| [165] | Matsumoto, Y.; Matsumara, Y.; Lio, A.; omezwa, T.Y. Bull. Chem. Soc. Japan 1970, 43, 1496. |
| [166] | Moreno, E.; Ancizu, S.; Pérez-Silanes, S. Eur. J. Med. Chem. 2010, 45, 4418- 4426. |
| [167] | Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D. et al. Chem Biol Drug Des 2011, 77, 255-267. |
| [168] | Yingjun, X.; Fanhong, W.u.; Zhiyi, Y.; Minmin, Z.; Sheng, J. Molecules 2011, 16, 6894-6901. |
| [169] | Zarranz, B.; Jaso, A.; Lima, L. Brazilian J. Pharma. Sciences 2006, 42, 358-361. |
| [170] | Ancizu, S.; Castrillo, N.; Pérez-Silanes, S ; et al. Molecules 2012, 17, 7737-7757. |
| [171] | Liu, G.; Zhou, Y. u.; Lin, D.; Wang, J. ACS Comb. Sci. 2011, 13 (3), 209-213. |
| [172] | Moustafa, O. S.; Badr, M.Z.A.; El-Emary, T.I. Bull. Korean Chem. Soc. 2002, 23, 567-570. |
| [173] | Bansal, R.K. 3rd ed. New Age International Pvt. Ltd., Heterocyclic chemistry. 2005, 28, 464-472. |
| [174] | Landquist, J. K. J. Chem. Soc. 1953, 2816-2821. |
| [175] | Taranu, I. ; Popa, I.; Dragos, A. ; Vlatanescu, N. ; Buzatu, D. Chem. Bull. "Politehnica" Univ. (Timisoara) 2008, 53(67) ,1-2,192-195. ; Elina, A. S. ; OYu, M. Gen. Chem. (USSR) 1955, 25,145. |
| [176] | Taranu, B.O.; Popa, I.; Dragos, A.; Taranu, I.; Dobrescu, M.C.; Vlatanescu, N.V. Chem. Bull. "Politehnica" Univ. (Timisoara) 2010, 55(69), 2,175-179.; Kimura T, Yakugaku Zasshi, 1957, 77,891. |
| [177] | Maxer, A.; Salzmann, U.; Hofler, F. Helv. Chim. Acta.1971, 54, 2507. |
| [178] | Takaski, H.; Otomasu, H. Chem. Pharm. Bull., 1970, 18, 22-25. |
| [179] | Srinivas, M.; Tejasri, A.; Anjaneyulu, N.; Satyanarayana, K. Inter. J. Pharma Sciences 2013, 3, 142-146. |
| [180] | Baritrop, J. A.; Richards, C.G.; Russel, D. M. J. Chem. Soc.1959, 1423. |
| [181] | Hammer, J.; Halliday, R. E. J. Org. Chem. 1963, 28, 2488-2488. |
| [182] | Ribel, W. J.; Blach, T. N. J. Org. Chem. 1959, 24, 205-207. |
| [183] | Rao, K.V.; Jackman, D. J. Heterocycl. Chem. 1973, 10, 213-215. |
| [184] | DeSelms, R. C.; Greaves, R. J.; Schleigh, W. R. J. Herterocycl. Chem. 1974, 11, 595. |
| [185] | Smith, H.; Habib, M. S.; Iqbal, M.; Qureshi, M. I. J. Chem. Soc. 1964, 4053-4056. |
| [186] | Mamedov, V.A.; Khafizova, E.A.; Gubaidullin, A. T.; et al. Russian Chemical Bulletin, International Edition 2011, 60, 368-372. |
| [187] | Puratchikody, A.; Natarajan, R.; Jayapal, M.; Doble, M. Chem Biol Drug Des 2011, 78,988-998. |
| [188] | Thirupathaiah, T.; kranthi k.; Laxminarayana, E.; Thirumala, C. M. Inter. J. Chem Tech Research, Coden (USA): IJCRGG, 2010, 2(4), 1987-1991. |
| [189] | Sarodnick, G.; Heydenreich, M.; Linker, T.; Kleinpeter, E. Tetrahedron 2003, 59, 6311-6321. |
| [190] | Yurii, A.; Sayapin, V. N. K.; Serguei, V. V.; et al. Arkivoc, 2009, iv, 46-56. |
| [191] | Kuntal, M. Y. K. A. Med Chem Res 2011, 20, 300-306. |
| [192] | Mamedov, V. A.; Kalinin, A. A.; Gorbunova, E. A.; Bauer, I.; Habicher, W. D. Russian J. Organic Chem. 2004, 40, 1041-1046. |
| [193] | El’chishcheva, N.V.; Shklyaev, Yu. V.; Vnutskikh, Zh. A.; Chekryshkin, Yu. S.; Odegova, T. F. Pharmaceutical Chemistry J. 2012, 46,346-350. |
| [194] | Wang, M.; Zhang, T.T.; Gao, J.J.; Liang, Y. Chem. Heterocyclic Comp. 2012, 48(6), 897-902. |
| [195] | Mamedov, V.A.; Kalinin, A.A.; Gubaidullin, A.T.; Isaikina, O.G.; Litvinov, I. A. Russian J. Organic Chem. 2005, 41, 599-606. |
| [196] | Mamedov, V.A.; Murtazina, A. M.; Gubaidullin, A.T. Russian Chemical Bulletin, International Edition 2010, 59, 1645-1655. |
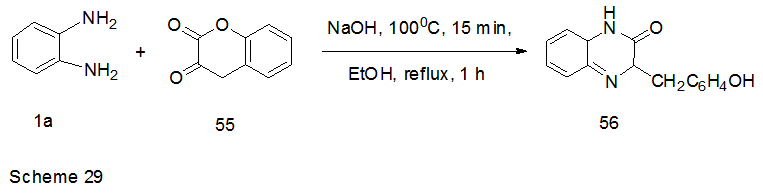
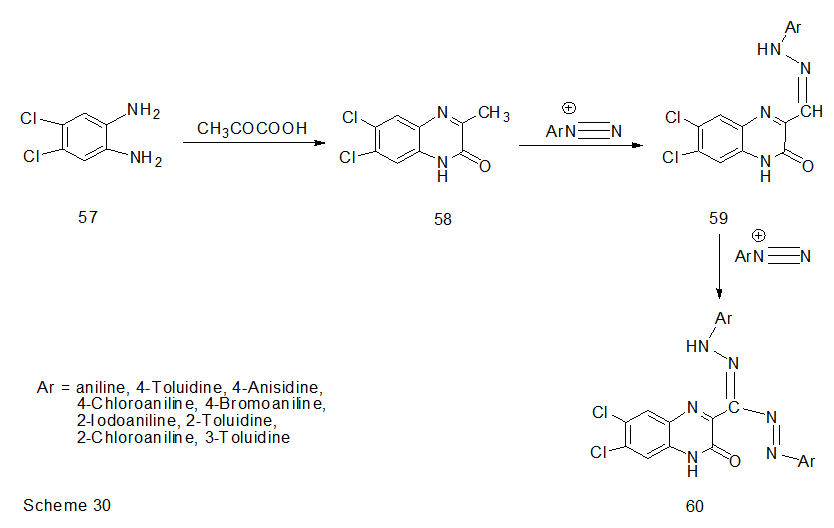
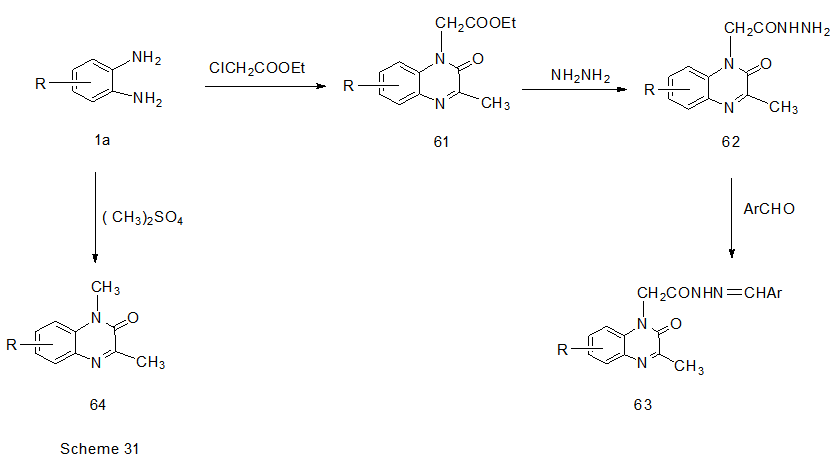
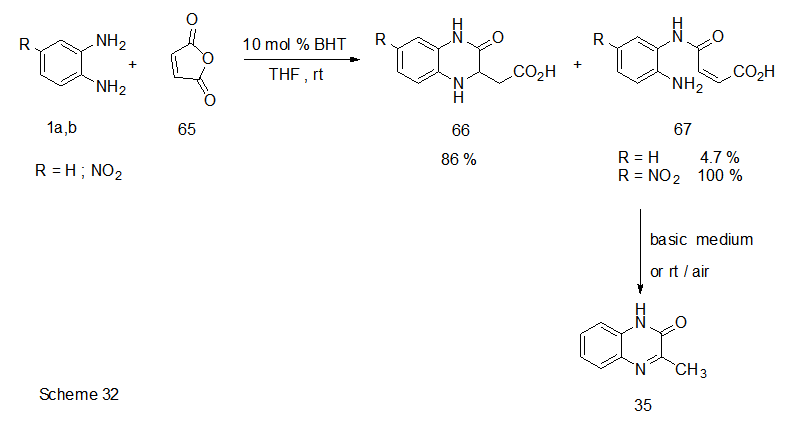
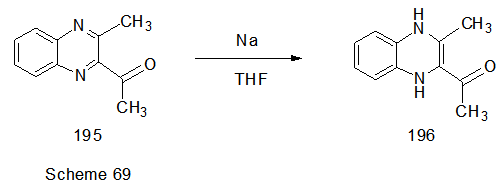
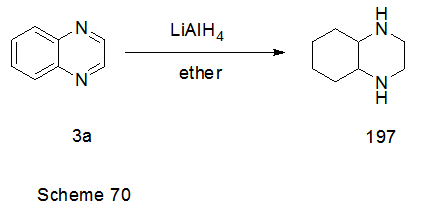
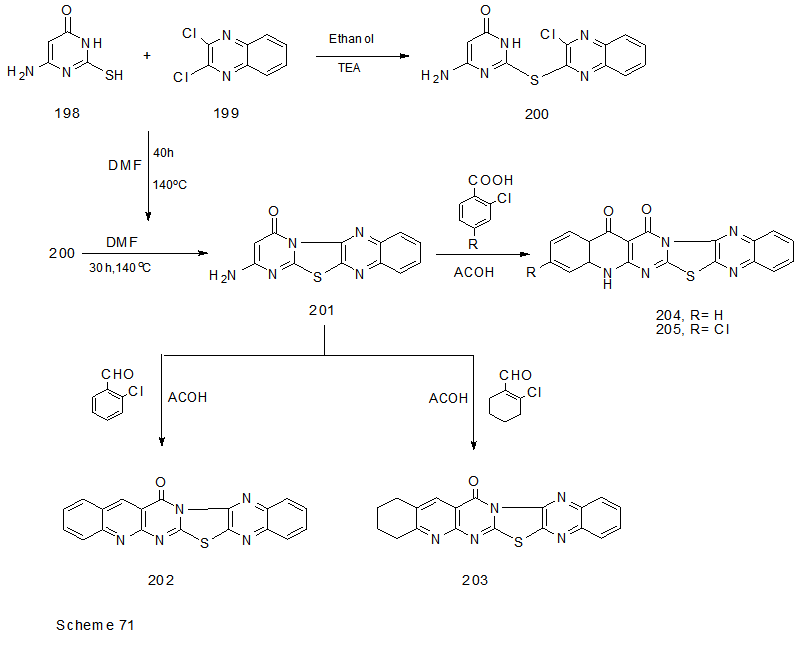
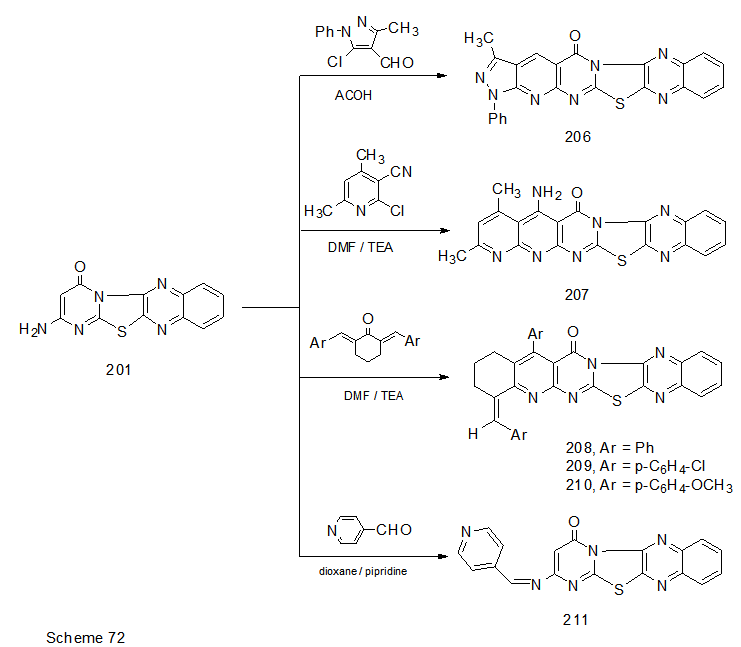
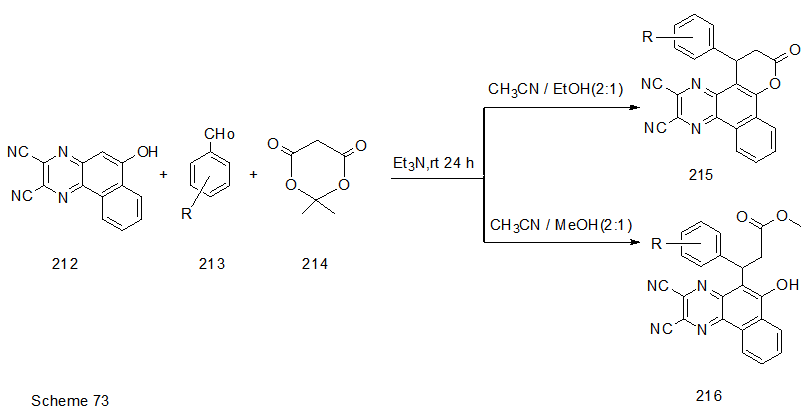
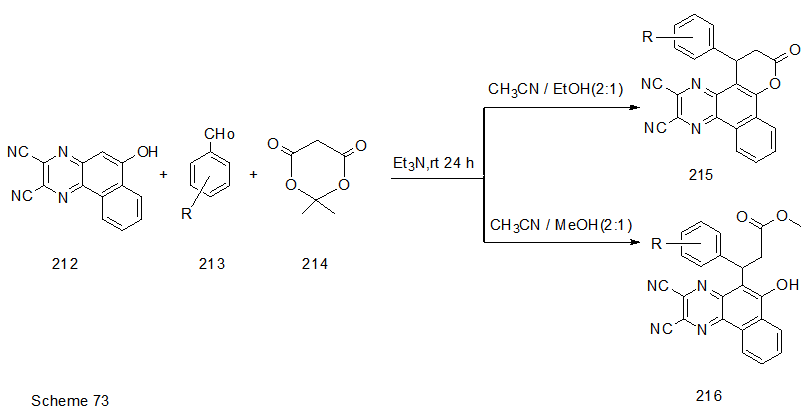
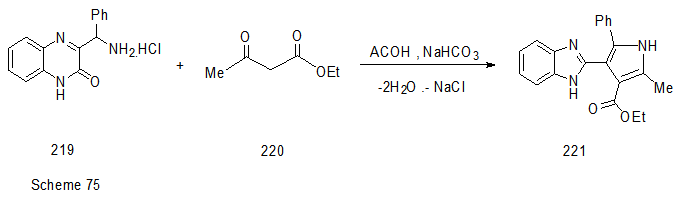
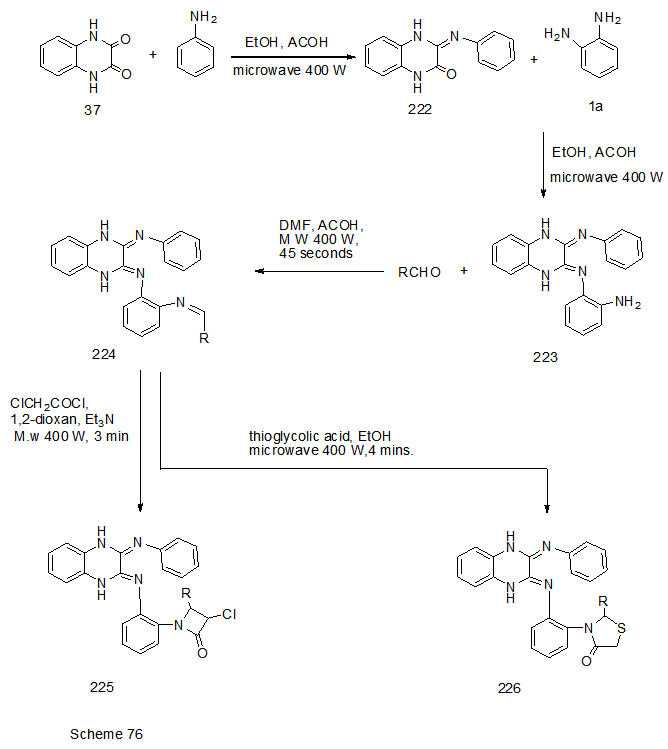
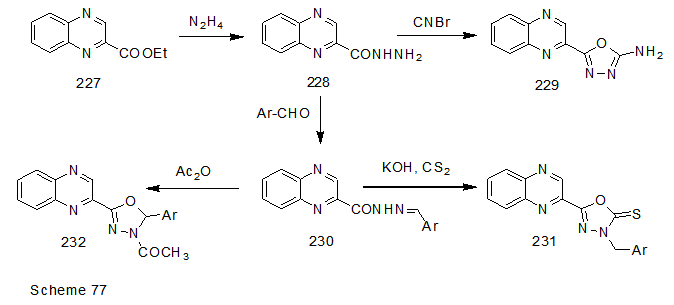
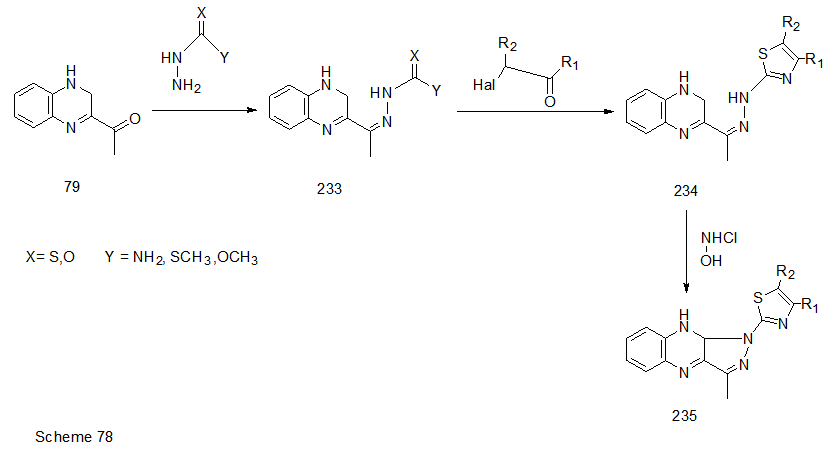
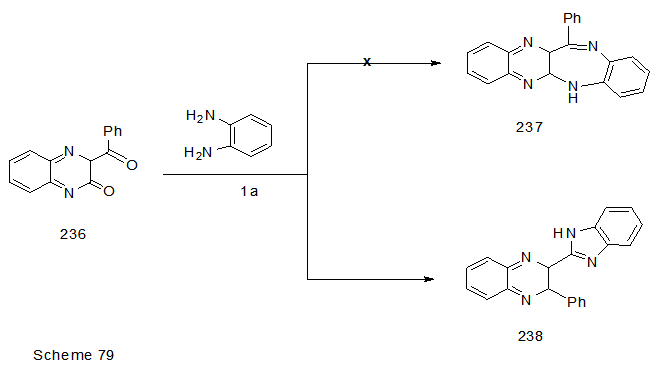
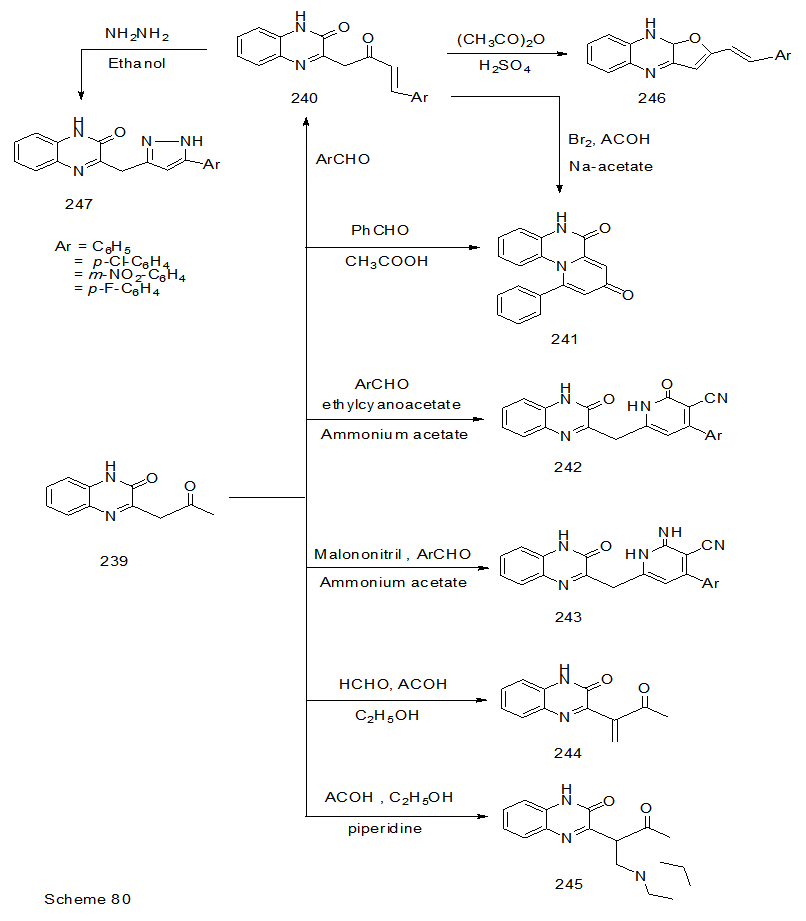
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML