-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2014; 4(2): 52-62
doi:10.5923/j.ajoc.20140402.03
Synthesis and Characterization of Some Novel 4-Thiazolidinones and Isoxazolines Derived from Thiosemicarbazones
Ahmed N. Ayyash1, Hamed J. Jaffer1, Jumbad H. Tomma2
1Department of Chemistry, College of Science, University of Al-Mustansiriyah, Baghdad, Iraq
2Department of Chemistry, College of Education for Pure Science, Ibn Al-Haitham, University of Baghdad, Baghdad, Iraq
Correspondence to: Ahmed N. Ayyash, Department of Chemistry, College of Science, University of Al-Mustansiriyah, Baghdad, Iraq.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
A Novel compounds of 1,3-thiazolidine-4-ones and isoxazolines fused with thiazolidine ring have been synthesized from some thiosemicarbazones. The thiosemicarbazones were prepared by the reaction of thiosemicarbazide with diffrent aldehydes and ketones. A2-substituted-1,3-thiazolidine-4-oneswere synthesized by the reaction of thiosemicarbazones with chloroacetic acid in presence of anhydrous sodium acetate. The 4-thiazolidinones have a double bond at the position -5 of the 4-thiazolidinone ring. The double bond was inserted by the reaction of thiazolidinones with benzaldehyde in piperidine. Hydroxylamine hydrochloride was used to convert the later compounds to isoxazolines fused with thiazolidine ring. Furthermore, 4-thiazolidinones containing N-acetyl group were obtained by the reaction of 4-thiazolidinones with acetic anhydride. The structures of newly synthesized compounds were established on the basis of spectroscopy data.
Keywords: 4-Thiazolidinone, FusedIsoxazoline, Amides, Thiosemicarbazone
Cite this paper: Ahmed N. Ayyash, Hamed J. Jaffer, Jumbad H. Tomma, Synthesis and Characterization of Some Novel 4-Thiazolidinones and Isoxazolines Derived from Thiosemicarbazones, American Journal of Organic Chemistry, Vol. 4 No. 2, 2014, pp. 52-62. doi: 10.5923/j.ajoc.20140402.03.
Article Outline
1. Introduction
- Many thiazolidinone and isoxazoline derivatives demonstrated a wide spectrum of biological activities [1-4]. These activities are include anti-bacterial, anti-fungi, anti-convalsant and anti-inflatmmatory. The 4-thiazolidinone class represent an important analogue to thiazolidine heterocyclic compounds [5]. Derivatives of 4-thiazolidinone were synthesized by various methods. However, aconventional method for such synthesis was frequently used. It is involve the cyclo-condensation reaction one-pot method was convenient for synthesis of 4-thiazolidinone. This method includes the reaction of enaminones with ethyl-2-bromopropionate [7]. A common synthetic path for construction of iminothiazolidinones is the cyclization of thiourea or thiosemicarbazide derivatives with halo-esters or thioglycolic acids in presence of inorganic base in polar solvents. The cyclization reaction was carried out by conventional [8-14] or microwave irradiation techniques [15-18]. The classical method for synthesisisoxazoline derivatives involves a base catalyzedcondensation of chalcone with hydroxylamine hydrochloride between Schiff-bases and mercaptoacetic acid [6]. The in ethanol [19]. As a part of our interests towards developing novel heterocyclic compounds may be have useful biological activity, we plan to synthesis some new 4-thia -zolidinones and isoxazolines have significant structures derived from different thiosemicarbazides. Schemes (1) and (2).
2. Experimental
2.1. Instruments
- Melting points were recorded using SMP 30 melting point instrument (Stuart, Germany), and they are uncorrected. 1HNMR spectra were recorded in DMSO-d6 on Bruker (400 MHZ) DMX-500 NMR spectrophotometer, at Al-albayt university, Jordan. The chemical shifts were recorded as values in ppm using tetramethylsilane (TMS) as internal standerd. FT-IR spectra were recorded as KBr discs on Shimadzu FT-IR 8400S spectrophotometer. All reactions and the purity of the synthesized compounds were monitored by using TLC (silica gel).The mass spectra were recorded on Shimadzu model GCMS QP 1000EX gas chromatography -MS apparatus (Japan) at the department of chemistry college of science, university of Al-Mustansiriya (Baghdad-Iraq).
2.2. General Procedures
- All new heterocyclic derivatives were synthesized according to schemes (1) and (2).
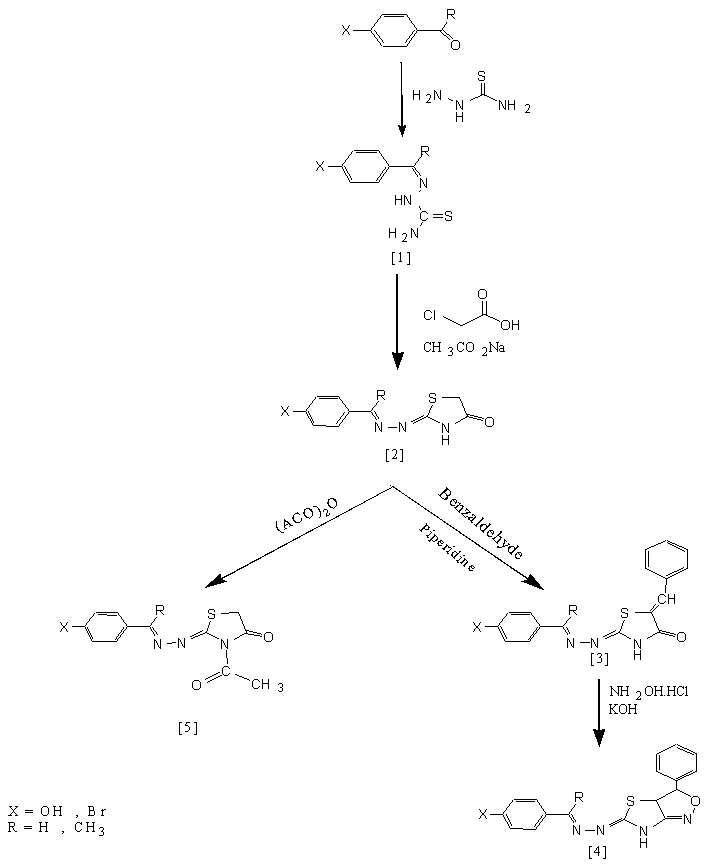 | Scheme (1). Synthetic route for target compounds [4] and [5] |
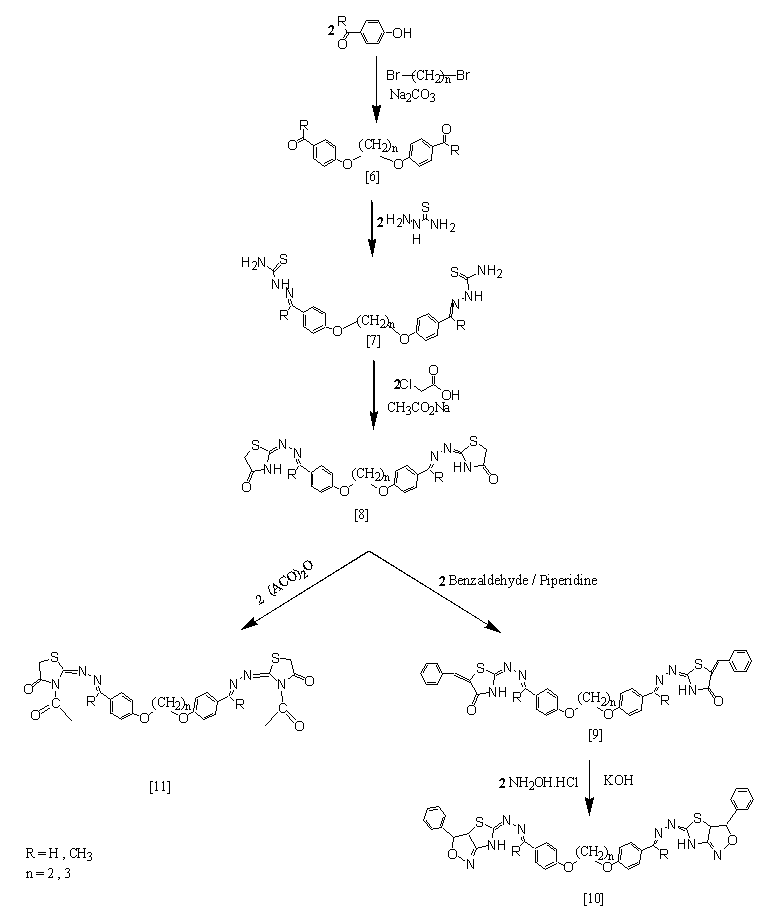 | Scheme (2). Synthetic route for target compounds [10] and [11] |
2.2.1. Preparation of thiosemicarbazones [1]a-d [20]
- Thio- semicarbazide (0.01mol) was added to a solution of 4-substitutedacetophenone (0.01mol) or 4-substituted- enzaldehyde(0.01mol) in absolute ethanol (20mL) and three drops of glacial acetic acid. The reactants were heated under reflux for 5 hrs .The product was cooled to room temperature and the solid was filtered, dried and recrystallized from ethyl acetate.
2.2.2. Synthesis of 2-(4-substituted-benzylidenehydra- zono)-1,3-thiazolidine-4-one [2]a-d
- A mixture consists of thiosemicarbazone [1] (0.01mol) in absolute ethanol (10mL) containing chloroacetic acid (0.01mol) and fused sodium acetate (0.03mol) was refluxed for 6 hrs. The resulted product was poured onto ice-water (100mL) and the formed precipitate was filtered, washed with water, dried and recrystallized from ethanol.
2.2.3. Synthesis of (5-benzylidene)-2-(4-substituted- benzylidenehydrazono)-1,3-thiazolidine-4-one [3]a-d
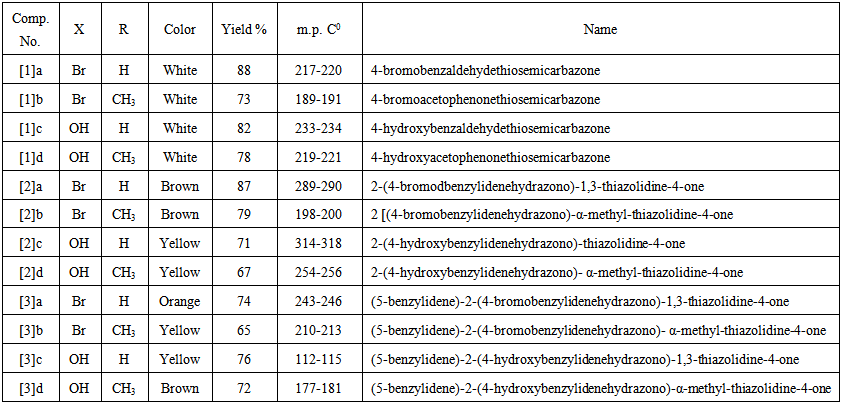
- A mixture of compound [2] (0.01mol) and benzalde- hyde(0.015mol) was fused in presence of piperidine(0.5 mL) for 3 hrs. The crude product was cooled to room temperature, and poured onto ice- water. The separated solid was filtered, washed, dried and recrystallized from acetone. The physical data of compounds [1]a-d – [3]a-d are listed in Table (1).
 | Table (1). Physical properties of compounds [1] to [3] |
2.2.4. Synthesis of 2-(4-substitutedbenzylidenehydra- zono)-3-phenyl-3,3a-dihydro-thiazolo [3,4-c]isoxazo -le [4]a-d
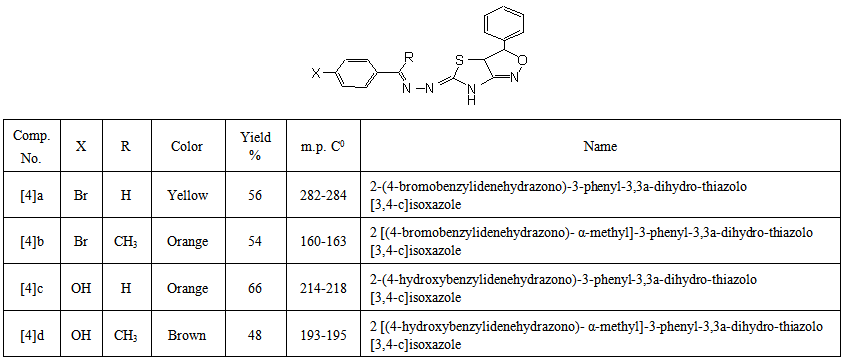
- A mixture of compound [3] (0.01mol), hydroxylamine hydrochloride(0.01mol) and potass -ium hydroxide(0.02 mol) in absolute ethanol (15mL) was heated under reflux for 16-18 hrs., (Monitored by TLC). The crude product was left to cooled to room temperature, then poured onto ice- water. The formed solid wasfiltered, washed with water, dried and recrystallized from ethanol. The physical properties of these compounds are listed in Table (2).
 | Table (2). Physical properties of compounds [4] a -c |
2.2.5. Synthesis of 3-acetyl-2-(4-substitutedbenzyli- denehydrazono)-1,3-thiazolidine-4-one [5]a-d
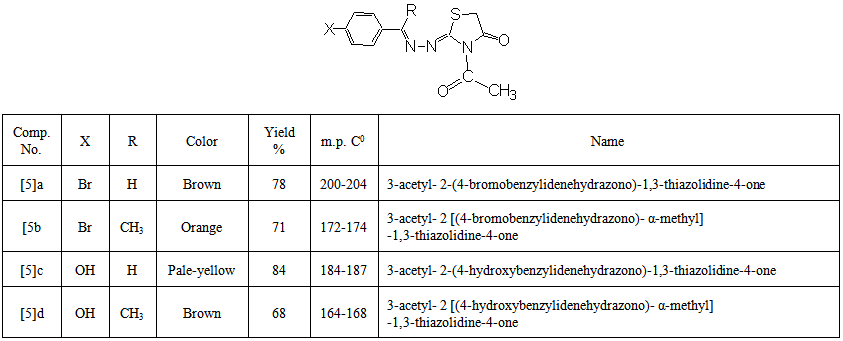
- Asolution of compound [4] (0.001mol) in acetic anhydride (3mL) was heated under reflux for 4 hrs. The mixture was cooledtoroom temperature, then poured onto ice-water. The separated solid was filtered, washed with excess water, dried andrecrystallized from ethanol. The physical properties of these compounds are given in Table (3).
 | Table (3). Physical properties of compounds [5] a–d |
2.2.6. Synthesis of polymethylene-bis-4-oxybenzal-dehydes and polymethylene-bis-4-oxyacetophenones [6]a-d
- To a mixture of 4-hydroxylbenzaldehyde (0.02mol) or 4-hydroxyacetophenone (0.02mol) and 1,2-butane or 1,3-dibromopropane (0.01mol) in N,N-dimethylformamide (15mL), anhydrous sodium carbonate (0.025mol)was added. The mixture was heated under reflux with continuous stirring for 4hrs. The crude product was cooled to room temperature, then poured onto ice-cold water and left in therefrigerator overnight. The resulted solid was filtered, washed with water, and recrystallized from ethanol.
2.2.7. Synthesis of polymethylene-bis-4-oxyphenyl –thiosemicarbazons [7]a-d
- Thiosemicarbazide(0.02mol) was added to a solution of compound [6] (0.01 mol) in absolute ethanol (20mL). Afew drops of glacial acetic acid was added and the reactants were heated under reflux for 5 hrs. The crude product was cooled to room temperature and the resulted solid was filtered, washed with water, dried and recrystallized from ethyl acetate.
2.2.8. Synthesis of polymethylene-bis- [2-(4-oxybenz- ylidenehydrazono)-1,3-thiazolidine-4-one] [8]a-d
- A mixture of thiosemicarbazone [7] (0.01mol), chloro- acetic acid (0.02mol) and fused sodium acetate (0.04mol) in absolute ethanol (10mL) was refluxed for 6 hrs. The crude product was cooled to room temperature, then poured onto ice-water. The resulted precipitate was filtered, washed with water, dried, and recrystallized from ethanol.
2.2.9. Synthesis of polymethylene-bis-[5-benzylidene-4- (2-imino-1,3-thiazolidine-4-one)-benzyledenehydra -zono-oxy] [9]a-d
- A mixture of compound [8] (0.01mol) and benzaldehyde (0.025mol) in piperidine (1mL) was heated in fusion temperature for 3 hrs. The crude product was cooled to room temperature, then poured onto- ice water. The separated solid was filtered, washed with water, dried, and recrystallized from acetone. The physical properties of the synthesized compounds [6]a-d- [9]a-d are listed in Table (4).
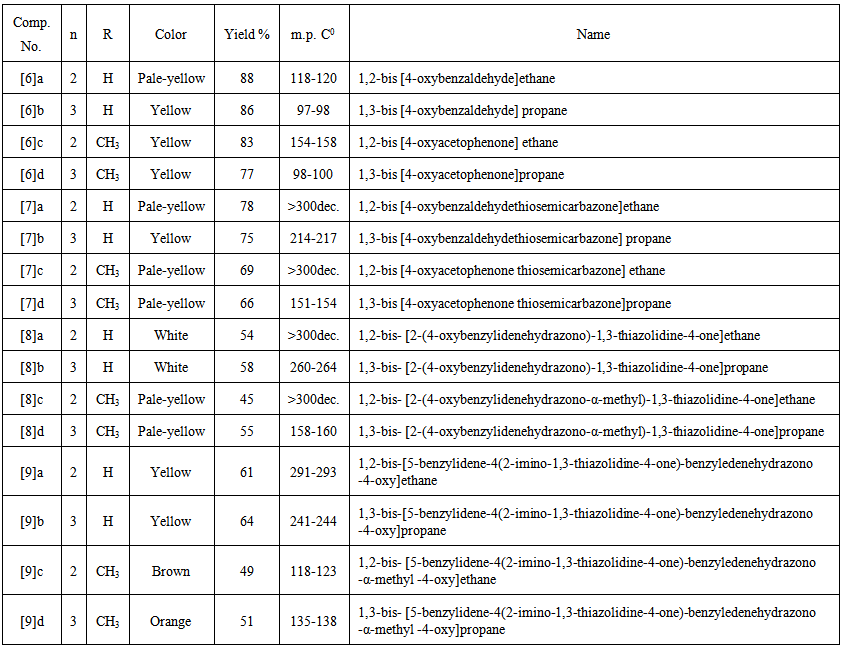 | Table (4). Physical properties of compounds [6] to [9] |
2.2.10. Synthesis of polymethylene-bis [4-(5-imino-3-phenyl-3,3a-dihydro-thiazolo [3,4c]isoxazole)-benzy- lidenehydrazono)-oxy]alkane [10]a-d
- A mixture of compound [9] (0.01mol), hydroxylaminehydro- chloride (0.02mol) and potassium hydroxide (0.04 mol) was dissolved in absolute ethanol(15 mL). The mixture was heated by reflux for 16-18 hrs., and the progress of the reaction was monitored by TLC. The crude product was left to cooled to room temperature and then poured into ice-cold water. The resulted solid was filtered, washed with water, dried, and recrystallized from ethanol. The physical data of these compounds are given in Table (5).
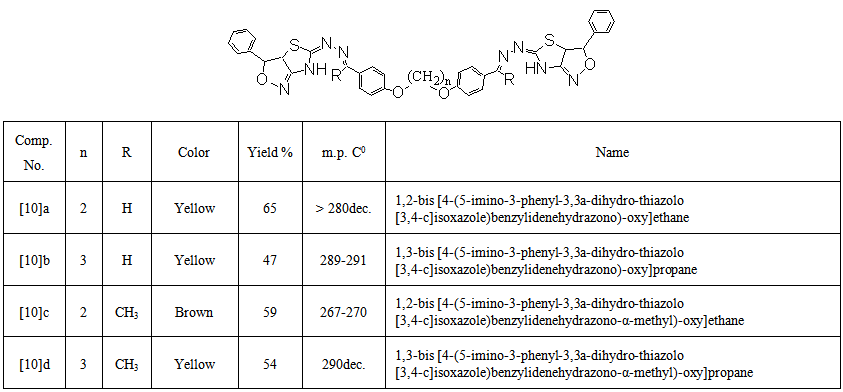 | Table (5). Physical properties of compounds [10] a-d |
2.2.11. Synthesis of polymethylene-bis-{4- [2-imino-N-acetyl-1,3-thiazolidine-4-one)benzyledenehydra- zono]-4-oxy}alkane [11]a-d
- A solution of compound [7] (0.001 mol) was dissolved in acetic anhydride (6 mL) and heated by reflux for 4 hrs. The crude product was left to cooled to room temperature, then poured onto ice-cold water. The separated solidwas filtered, washed with water, dried and recrystallized from ethanol. The physical data of these compounds are listed in Table (6).
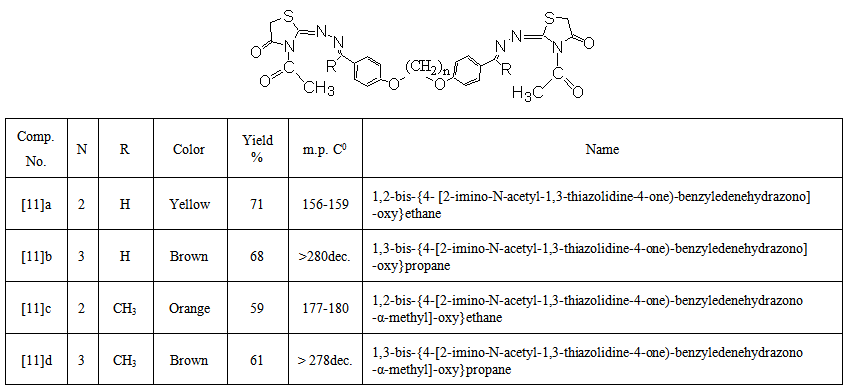 | Table (6). Physical properties of compounds [11] a-d |
3. Results and Discussion
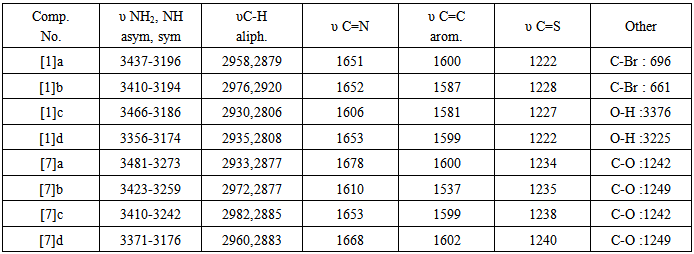
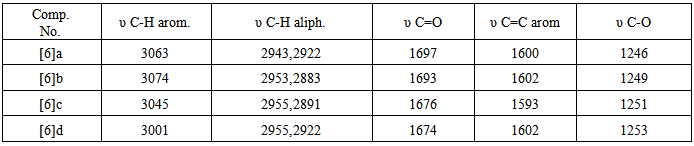
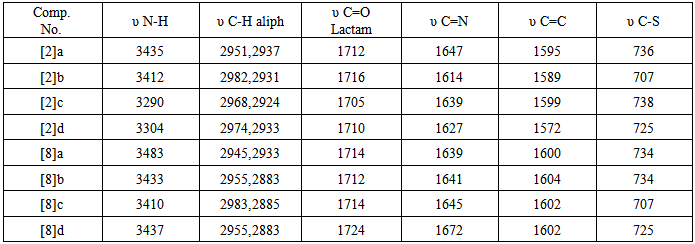
- In the present work, we report the synthesis of new derivatives of novel 4-thiazolidinone and isoxazoline fused with thiazoline ring. The target compounds were derived from thiosemicarbazone [1] and [7] which obtained from the reaction of different aldehydes and ketones with thiosemicarbazide in a good yields, schemes (1) and (2). The thiosemi-carbazones [1] and [7] were characterized by FT-IR spectroscopy and the characteristic absorption bands are listed in table (7). Three new stretching absorption bands at (3165- 3441 cm-1) are due to NH2 and NH groups, while the stretching absorption band due to C=N group appeared at (1650 cm-1). However, the spectra of these compounds showed the disappearances of carbonyl stretching absorption of the starting material (aldehydes and ketones). The resulted thiosemicarbazones [1] and [7] were cyclized successfully to 4-thiazolidinones [2] and [8] respectively, in good to moderate yields. The procedure includes the reaction of thiosemi-carbazones with chloroacetic acid and anhydrous sodium acetate in ethanol under reflux for 6 hrs. The disappearances of the starting materials was monitored by TLC. The cyclization mechanism may proceed as in scheme (3). The first step of the reaction includes the removal of proton from NH by sodium acetate resulted in conversion of the resulted intermediate to partially or totally to thiol form [21, 22]. The second step represent anucleophilic attack by thiol on carbon atom that bears a good leaving group (CH-Cl) will result in formation of new S-C bond. This step is followed by a nucleophilic attack by NH2 on carbon atom of carbonyl group resulted in formation of five member heterocyclic ring. The carbonyl group at position 4 of the hetrocyclic ring may be formed by losing one molecule of water. The FT-IRspectra of the products confirmed the formation of the 4-thiazoldinones [2] and [8], table (9). The spectra showed the disappearances of NH2 group bands of thiosemicarbazons [1] and [7] and the appearances of newcharacteristic bands at (3290-3483 cm-1) and (1683-1724 cm-1) belonging to the stretching vibration of N-H and C=O of lactam groups, respectively.
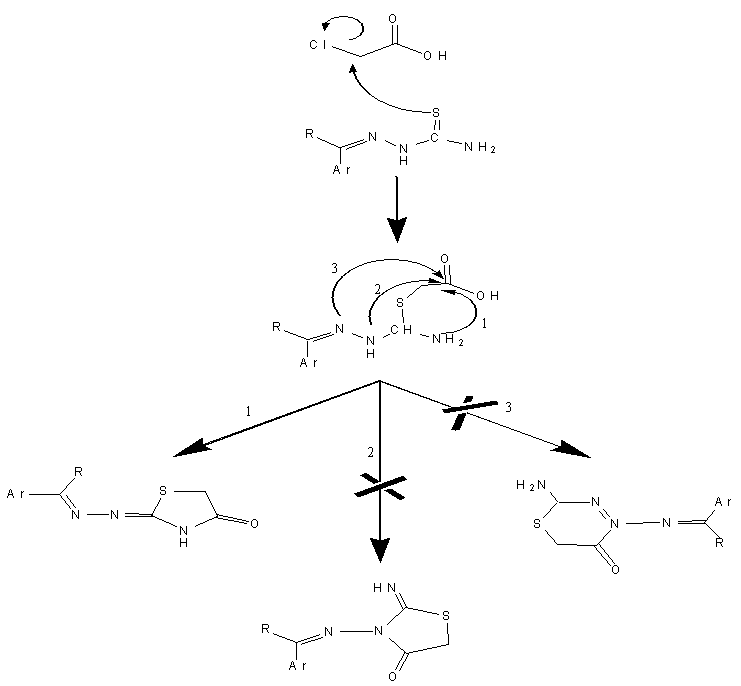 | Scheme (3). The suggested mechanism for synthesis 4-thiozolidinones |
|
|
|
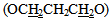 and two protons at C-5 of the five member ring, respectively. A third signal at δ (2.20) ppm due to two protons of methylene group
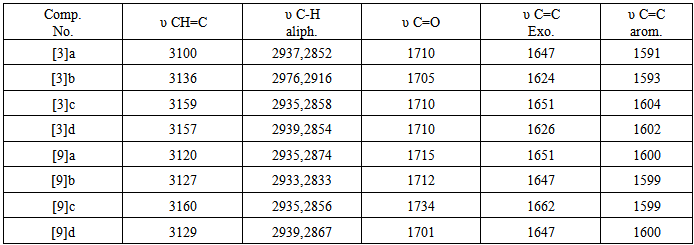
and two protons at C-5 of the five member ring, respectively. A third signal at δ (2.20) ppm due to two protons of methylene group 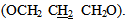 ppm. The mass spectrum of 4-thiazolidinone [2] a exhibited m/z = 298(M+) in addition to most significant fragments of this compounds are: 299(M+1), 198, 184, 170, 157, 142, 116, 102, 89 (base peak), 62. The second step of our plan is to introducea double bond at position 5 of the thiazolidinone ring. to give alkene compounds [3] and [9]. This step was carried out by fusion reaction of thiazolidinone [2] or [8] with benzaldehyde in presence of piperidine. The piperidinerole as a base was to remove the most acidic proton at position 5 of the ring. The resulted carbanion would easy attack the carbon of the carbonyl group of the benzaldehyde to produce the alkene [3] or [9]. The structure of the resulted products were confirmed by their FTIR spectra which showed a stretching vibration band for olefinic double bond (C=C) in the region (1624-1662 cm -1). The most characteristic absorption bands of the products are listed in table (10). furthermore, 1HNMR spectra of compound [3]d (in DMSO as solvent ) showed signals atδ7.0-7.9 ppm due to nine aromatic protons and one olefinicproton of CH= group, three sharp singlet singals at δ (12.6) ppm and δ (7.84) ppm could be attributed to a proton of OH group, and NHgroup, respectively. 1HNMR spectrum of compound [9] b (in DMSO as solvent) showed acomplicatedsignals between in the region δ 7.02-8.54 ppm due to 22 protons. Eighteen of them are aromatic protons, two olefinicprotons (CH=) and two protons of imine groups (CH=N). Also the spectrum showed a triplet signal at δ 4.24-4.28equivalent to four protons of two alkoxy group
ppm. The mass spectrum of 4-thiazolidinone [2] a exhibited m/z = 298(M+) in addition to most significant fragments of this compounds are: 299(M+1), 198, 184, 170, 157, 142, 116, 102, 89 (base peak), 62. The second step of our plan is to introducea double bond at position 5 of the thiazolidinone ring. to give alkene compounds [3] and [9]. This step was carried out by fusion reaction of thiazolidinone [2] or [8] with benzaldehyde in presence of piperidine. The piperidinerole as a base was to remove the most acidic proton at position 5 of the ring. The resulted carbanion would easy attack the carbon of the carbonyl group of the benzaldehyde to produce the alkene [3] or [9]. The structure of the resulted products were confirmed by their FTIR spectra which showed a stretching vibration band for olefinic double bond (C=C) in the region (1624-1662 cm -1). The most characteristic absorption bands of the products are listed in table (10). furthermore, 1HNMR spectra of compound [3]d (in DMSO as solvent ) showed signals atδ7.0-7.9 ppm due to nine aromatic protons and one olefinicproton of CH= group, three sharp singlet singals at δ (12.6) ppm and δ (7.84) ppm could be attributed to a proton of OH group, and NHgroup, respectively. 1HNMR spectrum of compound [9] b (in DMSO as solvent) showed acomplicatedsignals between in the region δ 7.02-8.54 ppm due to 22 protons. Eighteen of them are aromatic protons, two olefinicprotons (CH=) and two protons of imine groups (CH=N). Also the spectrum showed a triplet signal at δ 4.24-4.28equivalent to four protons of two alkoxy group 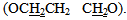 A multiple signal appeared at δ2.14-2.24 ppm due to two aliphatic protons (OCH2
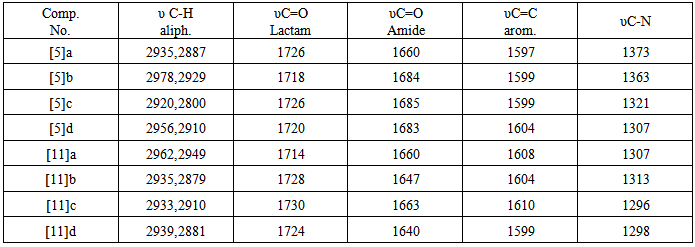
A multiple signal appeared at δ2.14-2.24 ppm due to two aliphatic protons (OCH2 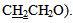 The mass spectrum of 4-thiazolidinoe [3] a exhibited m/z=287(M+) and another characteristic fragments of this compounds are an: 307,230,214,203,184, 134(base peak), 102,89,51. The second line of the present work is the synthesis of isoxazolines fused with thiazoline ring. This was achieved by cyclization of [3] or [9] with hydroxylaminehydrochloride in presence of base. The FTIR spectra of the products [4] and [10] confirmed the formation of these products and the data are listed in table (11). The significant remarks of these spectra are the disappearances of stretching vibration bands of C=CH and C=O groups of compounds [3] and [9]. A new bands appeared at 1662-1684 cm-1 and 1030-1068 cm-1 due to C=N and C-O respectively.
The mass spectrum of 4-thiazolidinoe [3] a exhibited m/z=287(M+) and another characteristic fragments of this compounds are an: 307,230,214,203,184, 134(base peak), 102,89,51. The second line of the present work is the synthesis of isoxazolines fused with thiazoline ring. This was achieved by cyclization of [3] or [9] with hydroxylaminehydrochloride in presence of base. The FTIR spectra of the products [4] and [10] confirmed the formation of these products and the data are listed in table (11). The significant remarks of these spectra are the disappearances of stretching vibration bands of C=CH and C=O groups of compounds [3] and [9]. A new bands appeared at 1662-1684 cm-1 and 1030-1068 cm-1 due to C=N and C-O respectively.
|
|
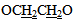 group and a signal at ppm δ 5.38 ppm for two protons of imine groups HC=N-. A singlet signal appear at δ 2.8 ppm are due to two protons at C-4. The mass spectrum of compound [10] b exhibited m/z = 714(M+-2H) and another most characteristicfragments of this compounds are: 338, 312(base peak), 307, 298, 230, 161, 134, 121, 102, 90, 51. Finally, the amide derivatives of 4-thiazolidinone [5] or [11] were produced from reaction under reflux between thiazolidinones [4] or [10] and acetic anhydride, respectively. The FT-IR data of compounds [5] and [11] are confirmed the structures of the products. The stretching vibration of amidic carbonyl (C=O) appeared at (1640-1685 cm-1), and the disappearances of absorption band of NH Lactam, table (12). 1HNMR spectrum of compound [5]a (in DMSO as solvent) showed pair of doublets signals in the region δ 7.62-7.38 ppm due to four aromatic protons, two sharp singlet signls at δ 6.74 ppm and δ 2.11 ppm could be attributed to proton of CH=N group and three protons of CH3 group, respectively. Also the spectrum showed a signals at δ 4.35 ppm due to two protons at C-5 of thiazolidinone ring. The mass spectrum of compound [5]b exhibited m/z = 354 (M+) and another most characteristic fragments of this compounds are :353 (M+-H) base peak, 340, 313, 298, 268, 240, 198 (base peak), 184, 156, 128, 102, 77, 51.
group and a signal at ppm δ 5.38 ppm for two protons of imine groups HC=N-. A singlet signal appear at δ 2.8 ppm are due to two protons at C-4. The mass spectrum of compound [10] b exhibited m/z = 714(M+-2H) and another most characteristicfragments of this compounds are: 338, 312(base peak), 307, 298, 230, 161, 134, 121, 102, 90, 51. Finally, the amide derivatives of 4-thiazolidinone [5] or [11] were produced from reaction under reflux between thiazolidinones [4] or [10] and acetic anhydride, respectively. The FT-IR data of compounds [5] and [11] are confirmed the structures of the products. The stretching vibration of amidic carbonyl (C=O) appeared at (1640-1685 cm-1), and the disappearances of absorption band of NH Lactam, table (12). 1HNMR spectrum of compound [5]a (in DMSO as solvent) showed pair of doublets signals in the region δ 7.62-7.38 ppm due to four aromatic protons, two sharp singlet signls at δ 6.74 ppm and δ 2.11 ppm could be attributed to proton of CH=N group and three protons of CH3 group, respectively. Also the spectrum showed a signals at δ 4.35 ppm due to two protons at C-5 of thiazolidinone ring. The mass spectrum of compound [5]b exhibited m/z = 354 (M+) and another most characteristic fragments of this compounds are :353 (M+-H) base peak, 340, 313, 298, 268, 240, 198 (base peak), 184, 156, 128, 102, 77, 51.
|
4. Conclusions
- In conclusion, new compounds of 4-thiazolidinone derivatives and fused-isoxazolines were synthesized in good yield derived from different thiosemicarbazones and they were characterized by different spectral studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML