-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2014; 4(2): 26-51
doi:10.5923/j.ajoc.20140402.02
Synthesis and Biological Applications of Hydroxamates
David I. Ugwu1, Benjamin E. Ezema1, Florence U. Eze1, Jude I. Ayogu1, Chidimma G. Ezema2, Daniel I. Ugwuja3
1Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Nigeria
2National Centre for Energy Research and Development, University of Nigeria, Nsukka, Nigeria
3Department of Chemical Sciences, Federal University, Wukari, Nigeria
Correspondence to: David I. Ugwu, Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Hydroxamates are physiologically active compounds. They have found applications as histone deacetylase inhibitors widely applied in cancer treatment such as vorinostat, belinostat, panobinostat and trichostatin A. There are hydroxamates with reported anti-HIV activity such as the hydroxyurea which acts as inhibitors of cellular enzyme ribonucleoside diphosphate reductase. Hydroxyurea are also used for treatment of chronic myelogenal leukemia, myeloproliferative syndromes and sickle cell anemia. Hydroxamates such as fosmidomycin and desferrioxamine B are potent antimalarial agent. Cipemastat, marimastat, periostat, ilomastat and batimastat are all hydroxamate-based inhibitors of matrix metalloproteinase and are by so used in management of cardiovascular diseases. The syntheses of various classes of hydroxamates and their mode of biological applications have been reviewed.The broad biological activities of hydroxamates and the need to improve on their synthetic routes informed the review of their synthesis and biological applications.
Keywords: Keyword Histone deacetylase inhibitors, Matrix metalloproteinase inhibitors, HIV, Hydroxamaates, Ribonucleoside diphosphate reductase
Cite this paper: David I. Ugwu, Benjamin E. Ezema, Florence U. Eze, Jude I. Ayogu, Chidimma G. Ezema, Daniel I. Ugwuja, Synthesis and Biological Applications of Hydroxamates, American Journal of Organic Chemistry, Vol. 4 No. 2, 2014, pp. 26-51. doi: 10.5923/j.ajoc.20140402.02.
Article Outline
1. Introduction
- Hydroxamates are class of organic compounds bearing the functional group RICON(OH)RII as organic residues and CO as a carbonyl group. They are amides where the hydrogen (H) atom of NH center has been replaced by an OH. They are otherwise called Weinreb amides. Hydroxamates are deprotonated product of hydroxamic acid and acts as excellent ligand. [1] Hydroxamates have high affinity for ferric ions that nature has evolved families of hydroxamic acids to function as no N-binding compounds (siderophores) in bacteria. [2] Hydroxamates of amino acids are effective inhibitors of amino peptidases. [3] Their mode of action results from the formation of bidentate ligand with active site of zinc. Hydroxamic acids have been the source of much biochemical interest in recent years reflecting the fact that they demonstrate a wide variety of biological activities. [4] Much of their activities are due to their chelating properties with metal ions, especially with transition metals, hence constituting a very important class of chelating agents with versatile biological activities. [5, 6] A number of synthetic routes are available for the preparation of hydroxamic acids, [7-12] but some are tedious, time consuming and costly as well. The reasonable way of producing hydroxamic acid derivative is the reaction of hydroxylamine with acid chlorides or esters. [13] Hydroxamic acids are capable of inhibiting a variety of enzymes, including ureases, [14, 15] peroxidises [16], and matrix metalloproteinases. [17, 18] These class of compounds are used in the design of therapeutics targeting cancer [19, 20] e.g. histone deacetylases (HDACs) inhibitors like vorinostat, belinostat, panobinostat and trichostatin A, [21] cardiovascular diseases [22] e.g. Marimastat, periostat, ilomastat etc., HIV [23] e.g. hydroxyurea, [24] Alzheimer’s, [25] malaria[26, 27] e.g. desferrioxamine B, fosmidomycin, siderophores [28] and allergic diseases. [29] The wide biological application of hydroxamates necessitates the review of its synthesis and biological applications.
2. General Synthesis of Weinreb Amides
- Hydroxamic acids are prepared usually from esters or acid chlorides or carboxylic acids.
2.1. Synthesis of Benzohydroxamic Acid (3)
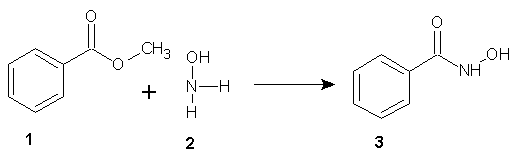
- The synthesis of compound 3 was achieved by reacting methyl benzoate (1) and hydroxylamine (2). [30]
 | Scheme 1 |
2.2. Synthesis of Hydroxamates Using N,N1,NII–trimethoxy-N,NI,NII-trimethyl Phosphorus Triamides 5
- Nui et al [31] reported the conversion of aromatic and aliphatic carboxylic acids, including sterically hindered substrates directly to hydroxamates using N,NI,NII-trimethoxy-N,NI,NII–trimethyl phosphorus triamide (5). On condensation of aromatic or aliphatic carboxylic acid (4) (0.01M) and compound (5) (0.005M) in toluene at 60℃ for 0.5 to 1 h, the hydroxamate (6) was obtained in excellent yield.
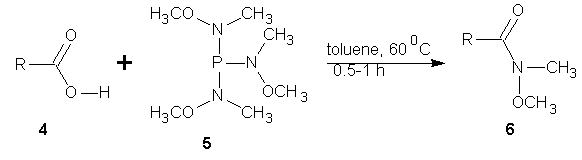 | Scheme 2 |
2.3. Ester Synthesis of Hydroxamate
- Riva et al [32] reported the transformation of methyl or ethyl carboxylic esters into the corresponding hydroxamic acid. To achieve this, the ester (0.5 M) in methanol and hydroxyl amine (10 eq) was reacted in the presence of sodium methoxide (1 eq). Following an optimization studies, they found that at 70℃ and 30 min, highest yield of the hydroxamate was obtained with high purity.
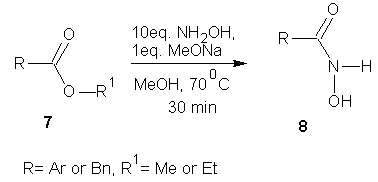 | Scheme 3 |
2.4. Microware Activated Hydroxamic Acid Synthesis
- Massaro et al [33] has shown that the reaction of esters with hydroxylamine in the presence of a base under microwave activation provides hydroxamic acids in good yield and high purity. The method has been success fully applied to enantiomerically pure esters without loss of stereochemical integrity.
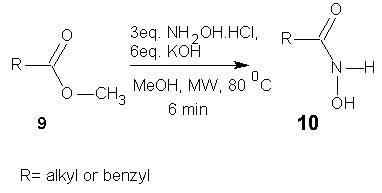 | Scheme 4 |
2.5. 1-Propanephosphonic Acid Cyclic Anhydride (T3P) Promoted Synthesis of Hydroxamic Acid
- 1-Propanephosphonic acid cyclic anhydride (T3P) promotes the synthesis of hydroxamic acids from carboxylic acids. [34] Application of ultra-sonication was showed to accelerate this conversion. Further, T3P has also been employed to activate the hydroxamtes leading to isocyanates via Lossen rearrangement [34].
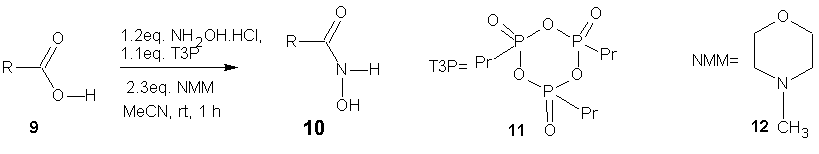 | Scheme 5 |
2.6. NHC–catalyzed Synthesis of Hydroxamic Acids
- N-Heterocyclic carbene (NHC) catalyzed amidation of a variety of aryl, alkyl, alkenyl and heterocyclic aldehydes with nitroso compounds is a powerful method for the synthesis of N-aryl hydroxamic acids in excellent yields. [35]
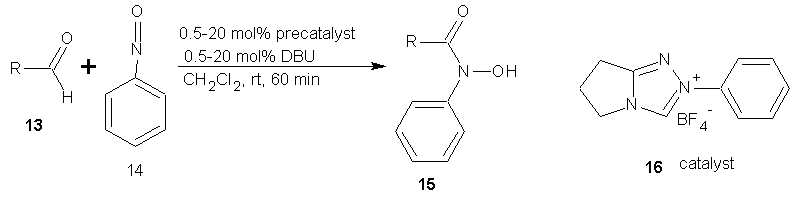 | Scheme 6 |
2.7. Chemoselective Esterificaiton Using Imidazole Carbamates
- Imidazole carbamates and ureas are used as chemoselective esterification and amidation reagents. A simple synthetic procedure allows the conversion of a wide variety of carboxylic acid to hydroxamates. [36]
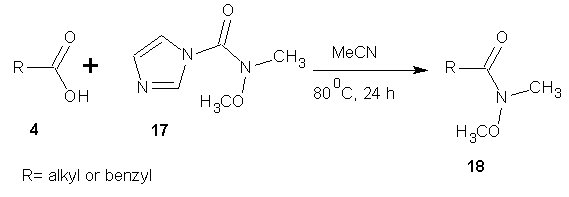 | Scheme 7 |
2.8. Synthesis of Weinreb Amides Using Triazime Intermediates
- De Luca et al [37] reported the successful large scale synthesis of weinreb amide through a convenient and simple one-flask method via 2-chloro-4,6-dimethoxy-1,3,5-triazine intermediate 20. The reaction of carboxylic acid (1eq) and 2-chloro-4,6-dimethoxy-1,3,5-triazine (1.2 eq) in the presence of N-methyl morpholine (3 eq.), THF at room temperature for 1-3 h gave the 4,6-dimethoxy-1,3,5-triazine intermediate 20, compound 20 upon treatment with substituted hydroxylamine (1 eq) at room temperature for 8 h gave the target product 21.There are many more general synthetic routes that have been reported and cannot be described for lack of space but are mentioned in this review. White et al [38] reported the synthesis of hydroxamates using Deoxo-fluor; Katritzky et al [39] reported the synthesis of N-alkyl, O-alkyl and O, N-dialkyl hydroxamic acids via acyl benzotriazole intermediates; Gissot et al [40] reported high yielding one step synthesis of hydoxamates from various un-activated esters (including enolizable esters and chrial α-amino acid esters and peptides); Woo et al [41] reported the conversion of sterically hindered carboxylic acids to N-methoxy-N-methyl amides using 1.1eq of methanesulphonyl chloride; Martinelli et al [42] reported the palladium catalysed amino carbonylation of anyl bromides into the corresponding Weinreb amides; Nemoto et al [43] also reported a one pot synthesis of α-siloxy weinreb amides from aldelydes using N,O-dimethly amine and a masked acyl cyamide reagent bearing a tert-butyl dimethyl silyl group.
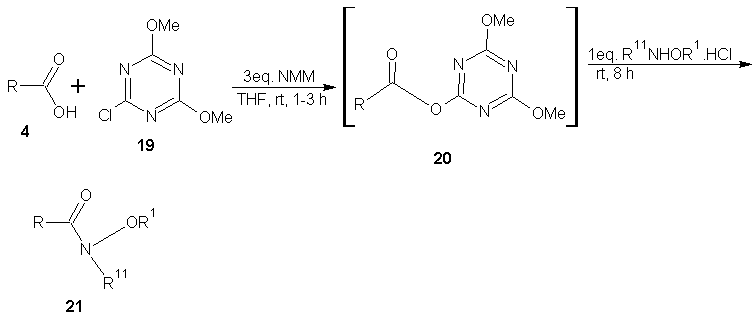 | Scheme 8 |
3. Synthesis of Anticancer Hydroxamates
- Most hydroxamates used in cancer chemotherapy acts as histone deacetylase (HDAC) inhibitors. Histone deacetylase are a group of enzymes that removes acetyl groups from the lysine residues on a histone. Removal of the acetyl groups known as hypo acetylation restores the normal positive change to the histone and therefore allows the DNA to condense and prevent transcription. This silencing can become permanent if the unprotected lysines are then methylated. HDAC performs the reverse process of histone acetyl coA to the lysines on the histone, inducing a state known as hyper acetylation. Hyper acetylation causes a decreased binding of the histones to DNA and leads to chromatin expansion, allowing transcription to take place. Hyper acetylation of histones increases the access of some transcription factors to nucleosomes thereby increasing RNA transcription. Histone deacetylase inhibitors (HDI) leads to hyper acetylation by blocking the function of histone deacetylase, therefore leaving the lysine amino acids acetylated from the histone acetyl transferase and ultimately increasing transcription. This process increases the amount of RNA present in the cell and their respective encoded proteins. [44]
3.1. Synthesis of Trichostatin A
- Trichostatin A was the first naturally occurring HDI to be discovered. It is also one of the most potent HDIs. It causes an increase in acetylated histones in a variety of mammalian tumor cell line. [45] Trichostatin A was shown to be a selective histone deacetylase inhibitor, reversibly inhibiting classes I, II and IV types of HDAC while not affecting class III. It exhibits an IC50 in the nanomolar range. [46]The first portion of the synthesis is shown in scheme 9. Protection of the commercially available hydro ester 22 with tertiary-butyldimethylsilyl chloride (TBDMS-Cl) gave silyl ester 23. The ester 23 on reduction with LiBH4 gave the alcohol. Oxidation of alcohol 24 under Swern condition [47] gave aldehyde 25 which was treated with an aryl Grignard reagent to produce alcohol 26. Alcohol 26 was treated with 2-methoxy propene and pyridinium p-toluenesulfonate (PPTS) to generate protected diol 27. The TBS group was later recovered with tetra- normal-butylammonium fluoride (TBAF) in THF to give alcohol 28 in 91% yields. The second portion of the synthesis is presented in schemes 10. Alcohol 28 was oxidized using Parikh-Doering conditions [48] to form aldehyde (29) following the findings of Smith et al. [49] Aldelyde 29 was reacted with (1-ethoxycarbonyl ethylidene) triphenylphosphorane in methylene chloride to produce ester (30) which was reduced to alcohol (31). Alcohol 31 was then oxidized using Parikh-Doering conditions [50] to form aldehyde (32) which was treated with (ethoxycarbonyl-methylene) triphenyl phosphorane in methylene chloride to provide ester (33). Finally, trichostatin A was obtained from 33 using the Mori and Koseki route [51] as presented in scheme 11. Ethyl ester 33 was treated with lithium hydroxide in methanol for 16 h at 45℃, then the pH of the reaction mixture was lowered to 3 with 1M HCl to give free acid (34), which was treated with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in 1,4-dioxane to give trichostatin acid (35) in 29% yield. Acid 35 was condensed with hydroxylamine (36), available in a 10% yield via a two-step sequence from N-hydroxyphthalamide to give the protected hydroxamic acid (37) in 63% yield. The protected acid 37 was then treated with amberlyst 15 in methanol to give trichostatin A (38).
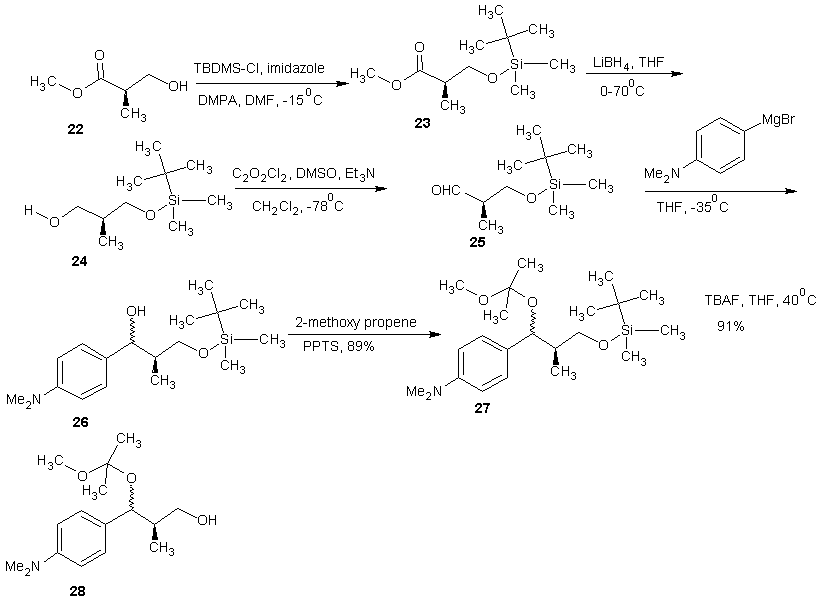 | Scheme 9 |
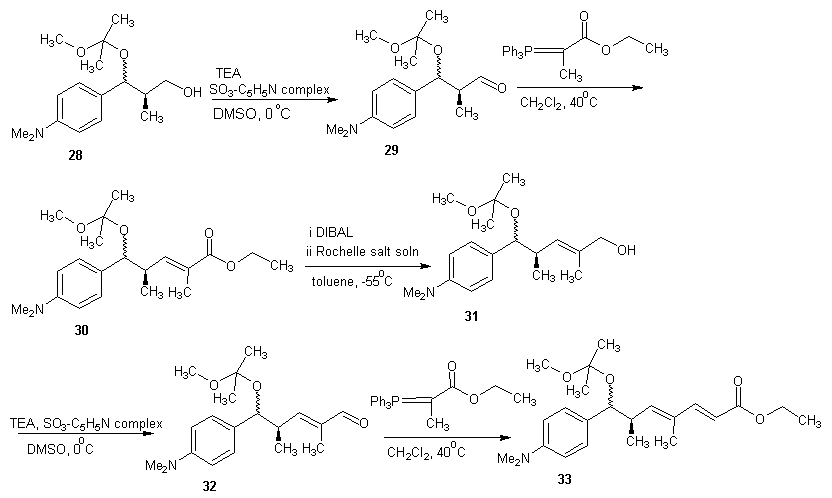 | Scheme 10 |
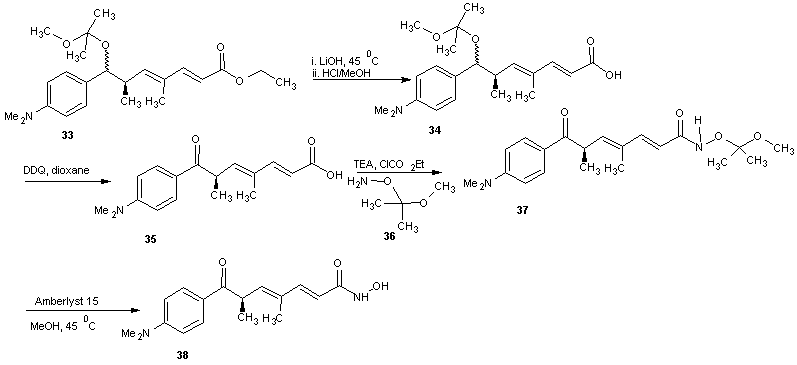 | Scheme 11 |
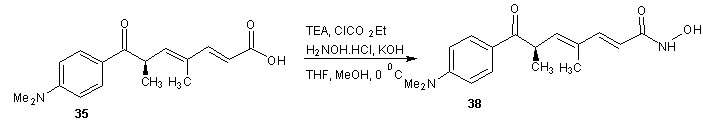 | Scheme 12 |
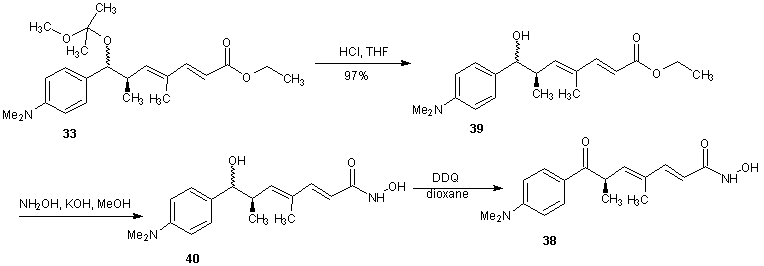 | Scheme 13 |
3.2. Synthesis of Vorinostat
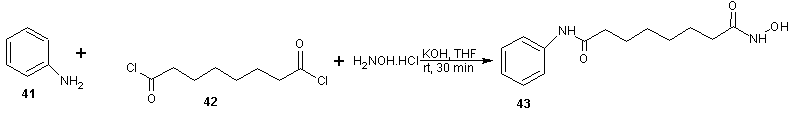
- Vorinostat, a histone deacetylase inhibitor, is currently marketed for the treatment of cutaneous T cell lymphoma (CTCL), a type of skin cancer. It is used for treating patients having a tumor characterized by proliferation of neoplastic cells. Process for the preparation of vorinostat and its form 1 crystalline polymorph have been disclosed in patent US 2004/0122101 and WO 2006/127319. The disclosed processes, comprising the preparation of vorinostat from suberic acid, are a cumbersome three steps of amidation of suberic acid with aniline, esterification of the mono amide product with methanol and finally reaction with hydroxylamine hydrochloride and sodium methoxide to afford vorinostat. This process is not very convenient as it involves elevated temperature, lengthy reaction times and has a low overall yield of around 23%. In addition, the intermediate products and final product are not very pure and require exhaustive purification steps. Four alternative processes to obtain vorinostat has been reported in US patent 5369108 by Vinayak et al [51] and illustrated in scheme14, 15, 16 and 17. In scheme 14, 16 and 17, amide formation was reported by the reaction of suberoyl chloride, anline and a third reactant. The third reactant was hydroxylamine hydrochloride, O-benzyl hydroxylamine and O-(trimethylsilyl) hydroxylamine in scheme 14, 16 and 17 respectively. The yield of vorinostat obtained in the three processes was almost (35%) in each case. In schemes 15, suberic acid monomethyl ester was converted to suberic acid monomethylester-monoacid chloride by treatment with oxalyl chloride and dimethyl formamide in benzene. The monomethylester–mono acid chloride formed was converted into the monoamide of suberic acid by treatment with aniline and subsequently potassium hydroxide. The suberic acid monoamide was treated with O-benzyl hydroxylamine and 1,3-dicyclohexyl carbodiimide (DCC) in pyridine, followed by hydrogenolysis to afford vorinostat. The product yields were from 35% to 65%.
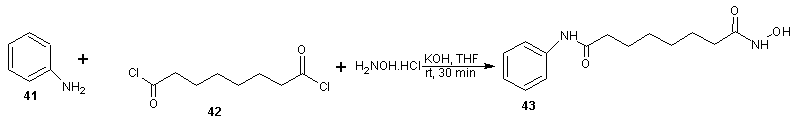 | Scheme 14 |
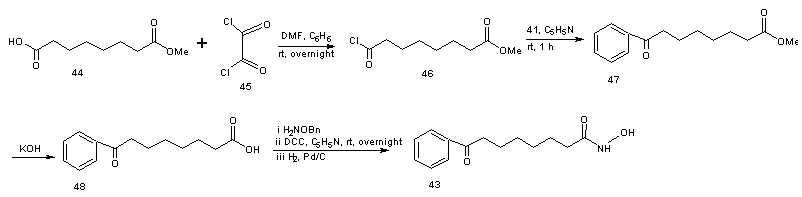 | Scheme 15 |
 | Scheme 16 |
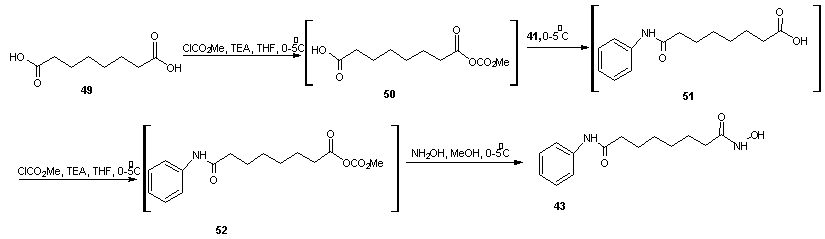 | Scheme 17 |
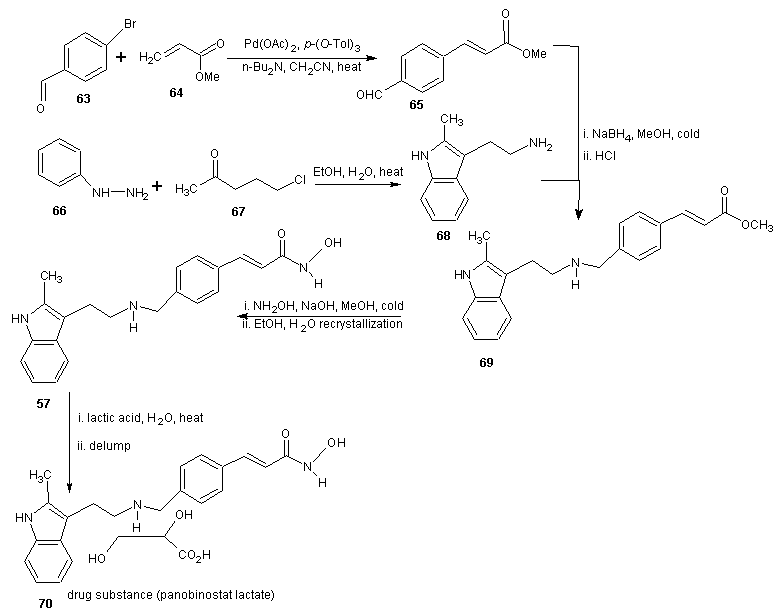
3.3. Synthesis of Panobinostat
- Panobinostat (LBH579) is an experimental drug developed by Novartis for the treatment of various cancers. It acts as a non-selective histone deacetylase inhibitor. [52] As at August 2012 it is being tested against Hodgkin’s Lymphoma, cutaneous T cell (CTCL) [53] and other type of malignant disease in phase III clinical trials, against myelodysplastic syndromes, breast cancer and prostate cancer in phase II trials and against chronic myelomonocytic leukemia in phase I trial. [54, 55]The synthesis is reported in scheme 18. 2-methyl indole-3-glyoxylamine (53) is reduced with LiALH4 to afford 2-methyltryptamine (54). 4-Formyl cinnamic acid is esterified with methanol and the resulting aldehyde ester (55) is reductively aminated with compound 54 in the presence of sodium cyanoborohydride (NaBH3CN) or sodium borohydride (NaBH4) to give an ester (56) which on treatment with aqueous hydroxylamine under basic condition gave the title hydroxamic acid (57).
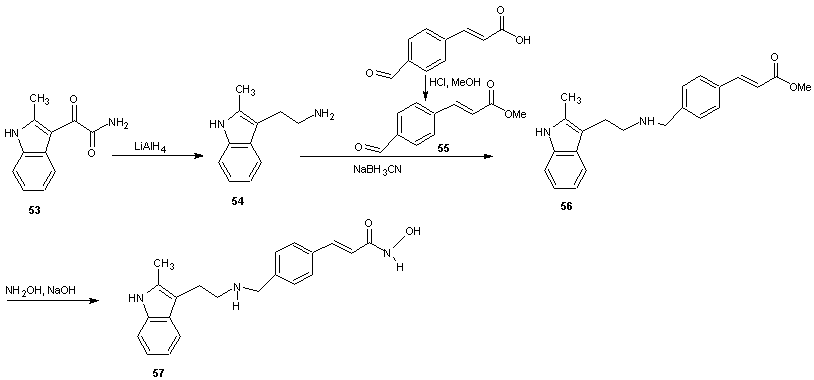 | Scheme 18 |
3.4. Synthesis of N-hydroxyl-3-[4-[{(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl}amino)methyl) phenyl)-2E-2-propenamide
- This is a derivative of panobinostat which has lost the methyl group on the indole moiety. This class of compounds is useful for treating proliferative diseases. The compounds have been found to be useful for treating breast cancer, genitourinary cancer, neuroblastoma, head and or neck cancer or bladder cancer or in a broad sense renal, brain or gastric cancer. The compounds were found to be selectively toxic or more toxic to rapidly proliferating cells than to normal cells. [57] In the synthesis, [58] the aldelyde (58) was reductively aminated to provide secondary or tertiary amines. 4-Methyl formyl cinnamate (59) on reaction with tryptamine (60) in the presence of sodium triacetoxy borohydride (NaBH(OAc)3) as a reducing agent using dichloroethane as solvent gave the amine (61). The amine 61 was converted to hydroxamic acid (62) by treatment with 50% aqueous hydroxylamine in a suitable solvent (e.g. THF in the presence of a base such as NaOH). Sodium borohydride (NaBH4) and sodium cyanoborohydride (NaBH3CN) in other solvents or solvent mixture in the presence of acid catalysts such as acetic acid or trifluoroacetic acid can also be used in place of NaBH(OAc)3.
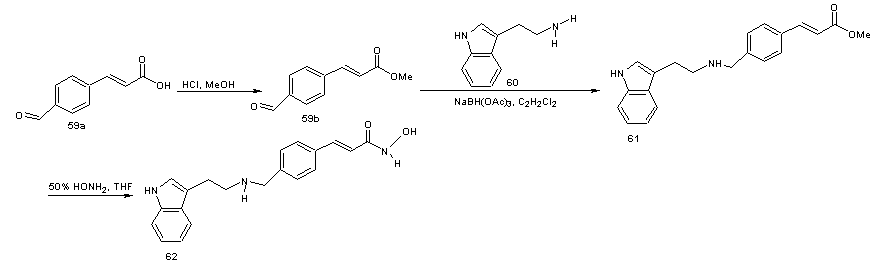 | Scheme 19 |
 | Scheme 20 |
3.5. Synthesis of Hydroxamic Acid Containing 1,4–Benzodiazepines
- The benzodiazepine derivative was synthesized by employing an intra-molecular Pd(0)-mediated ring opening of an acyl nitroso-derived cyclo adduct (71). The new hydroxamic acid containing benzodiazepine (72) was synthesized and has demonstrated biological activity in MCF-7 and PC-3 tumor cell lines. Subsequent N-O bond reduction of the hydroxamate has provided access to amide analogues (73) for structure activity relationship studies. [60]
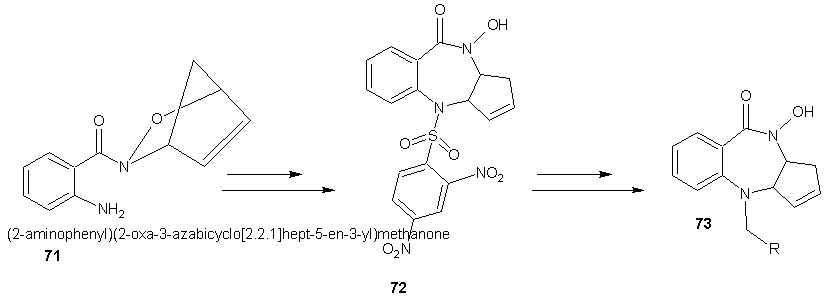 | Scheme 21 |
3.6. Synthesis of Polynucliar Diorganotin (IV) Complexes with Di-halogeno Benzohydroxamate Ligands
- The biological activity of organotin compounds is well known owing to their practical applications as fungicides, bactericides, biocides and pesticides. [61-65] Hydroxamic acids as inhibitors of 5-lipoxygenase can behave as strong bidentate O-donors with bioactivity. [66-70] The synthesis and activity of a series of 1:2 diorganotin (IV) aryl hydroxamate mononuclear complexes has shown that they can act as anticancer agents namely against gastric and liver carcinomas. [71-72] The agents were found to be difficult to formulate due to the low aqueous solubility subsequently, the fluoro complex [N-Bu2Sn[4-FC6H4C(O)NHO] was obtained and found to have a superior anti hepatocellular and nasopharyngeal activity in comparison with the chloro analogue and a broad spectrum of antitumor activity. [72, 73] Its IC50 values against two human tumor cell lines HCT-8 and Bel-7402 are 59 and 60 ng/mL respectively being considerably better than those of “cisplatin” and reflecting a marked effect of the para-halo-substituent in the benzohydroxamate ligand. Shang et al [74] also reported the in vitro cytotoxicity of diorganotin (IV) aryl hydroxamate complexes in human promyelocyticfina leukemic (HL-60), nasopharyngeal carcinoma (KB), hepatocellular carcinoma (Bel-7402) and gastric carcinoma (BGC-823) cell lines.Shang and co-workers [74] achieved the synthesis of organotin (IV) aryl hydroxamates simply by reacting an aromatic carboxylic ester with hydroxylamine hydrochloride to give the potassium salt which on treatment with acetic acid gave the aryl hydroxamates in excellent yield. The hydroxylamine was generated in situ using excessive KOH and NH2OH.HCl dissolved in dry ethanol.
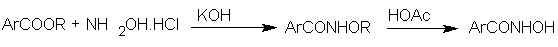 The complex, Bu2SnL2 was synthesized according to the following procedure: Di-n-butyltin oxide and an appropriate hydroxamic acid were reacted with refluxing in a mixture of toluene and ethanol (3:1) with azeotropic removal of water.
The complex, Bu2SnL2 was synthesized according to the following procedure: Di-n-butyltin oxide and an appropriate hydroxamic acid were reacted with refluxing in a mixture of toluene and ethanol (3:1) with azeotropic removal of water. The hydroxamates synthesized includes the following:
The hydroxamates synthesized includes the following: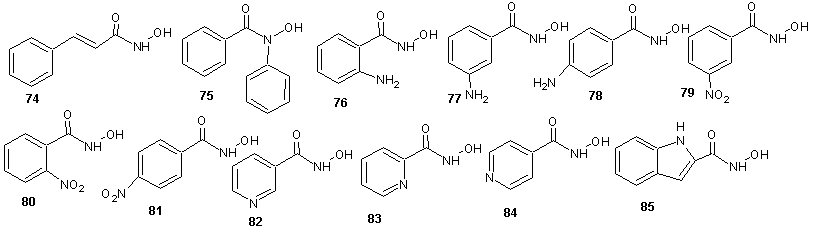 | Scheme 22 |
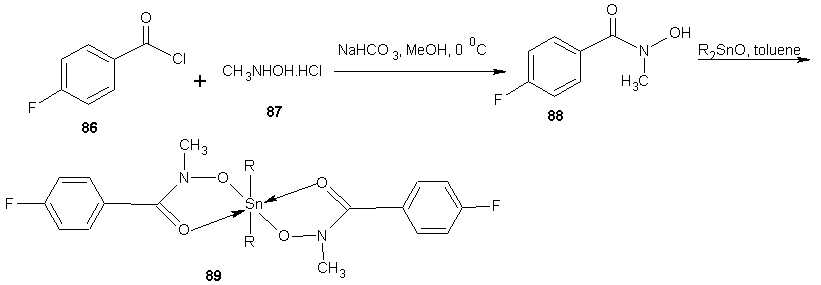 | Scheme 23 |
4. Synthesis of Anti-HIV Hydroxamates
- Efforts to find an effective anti HIV-1 chemotherapy have been focused on the development of chemicals that inhibit viral proteins, which are essential for HIV replication. The most important limitation of this therapeutic approach is the rapid generation of mutated quasi-species of HIV-1 resistant to those inhibitors. It has been suggested that a combination of antiviral chemotherapy with some inhibitors of cellular proteins may greatly improve the anti-HIV-I treatment. Among these proteins, the cellular enzyme ribonucleoside diphosphate reductase may be an important target, since this enzyme can be inhibited by compounds of the hydroxamate family such as hydroxyurea (HU). HU is a free radical quencher that inhibits the cellular enzyme ribonucleoside diphosphate reductase and in so doing, reduces the levels of deoxyribonucleotide. [77] Hydroxyurea has been used over the last 30 years for the treatment of human diseases such as chronic myelogenal leukemia, myeloproliferative syndromes and more recently sickle cell anaemia. [78-82] Moreover, HU inhibits HIV-1 DNA synthesis in activated peripheral blood lymphocytes by decreasing the amount of intracellular deoxynucleotides. Combination of hydroxyurea with the nucleoside analogue didanosine generated a synergistic inhibitory effect without increasing toxicity. [83-86]Hydroxyurea was first synthesized in 1869 by Dresler and stem from hydroxylamine and hydrogen cyanate; the industrial process is analogous. [87] HU may also be synthesized by reaction of ethyl carbonate with hydroxylamine wherein hydroxylamine displaces the ester to give the amide. [88] Hydroxyurea (91) is also prepared by reacting sodium cyanate (90) with hydroxylamine in the presence of a basic ion exchange resin amberlit.
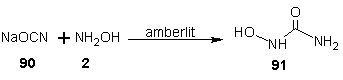
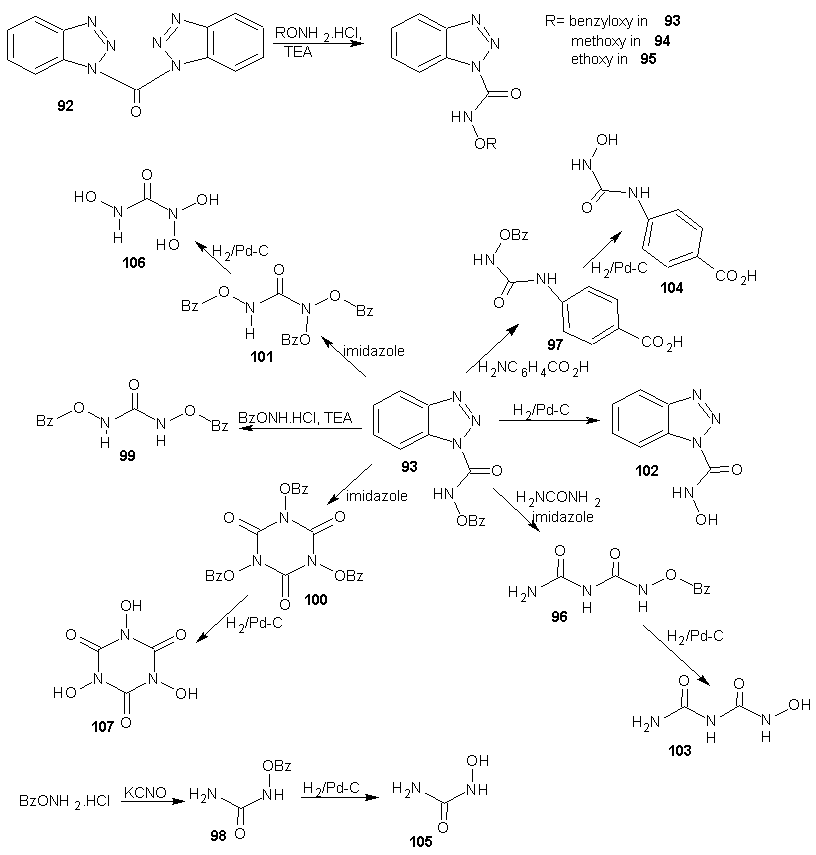 | Scheme 24 |
5. Synthesis of Antimalarial Hydroxamates
- Most hydroxamates that are used as antimalarial drugs acts as a chelator. The biological action has been attributed to chelation of internal iron pools [90] and in turn to interference with the supply of iron to different components, [91] possibly to ribonucleotide redutase. [91] In general, the speed of antimalarial action of any hydroxamate type chelator, the stage of specificity of the effects, and the growth arrest efficacy are largely a result of their membrane permeation properties and iron(III)-binding capacity [92] thus, the in vitro antimalarial action of the relatively hydrophilic desferrioxamine (DFO) B is manifested after 8 to 10 h of continuous exposure of Plasmodium falciparum trophozites to the drug (the 50% inhibitory concentrated in a 24 h culture was 30 mmol). [93] Less hydrophilic derivatives of desferrioxamine B such as N-terminal modified desferrioxamine [92] or a series of synthetic reversed siderophores [94] (RSFs) showed up to 10 fold higher potency as reflected in the speed of action and the 50% inhibitory concentration as well as a wider spectrum of activities at all developmental stage. [92] However, whereas the effects of DFO on trophozoites were relatively slow to develop, [91] but largely irreversible in nature, [92] those of RSFs were irreversible only on the ring stage but reversible in trophozoites. [93]
5.1. Synthesis of Desferrioxamine B
- Desferioxamine B is a polyhydroxamate iron chelator that is useful for reducing iron concentration in human blood plasma. It has the desirable property of high affinity for ferric ion (Ka = 1031) coupled with a very low affinity for calcium (Ka = 102). [94] Industrial scale fermentation process for producing desferrioxamine B uses the Streptomyces pilosus bacteria strain which produces a variety of polyhydroxamate compounds but predominantly desferrioxamine B, in a culture medium poor in iron. This is isolated from the fermentation broth as its hydrochloride salt which is not pharmaceutically acceptable. [95] Therefore it must be converted to the mesylate salt which is the approved desferrioxamine salt. The purification methods do not remove the fermentation products that are structurally related to desferrioxamine B efficiently. [96]
5.2. Production of Desferrioxamine B Using Corn Steep Liquor
- Desferrioxamine B is the major siderophore of Streptomyces pilosus. They are used clinically to treat disorders related to iron overload and pathological iron deposition in human. Corn steep liquor (CSL) is a by-product of corn wet-milling. It is an excellent source of organic nitrogen and important constituent of some culture media. The CSL is cheaper them other media and its availability is so easy. CSL virtually contains constituents that are naturally occurring nutritive materials such as crude proteins, amino acids, vitamins, reducing sugars, organic acids, minerals and other elemental nutrients. In their experiment, [97] lyophilized S. pilosus ATCC 19797 was cultured in several media such as malt yeast extract broth (MYB), nutrient broth (NB), Bertani (LB) and soybean. After 48 h incubation in shaker incubator at 29℃ and shaking at 150 rpm, the refreshed bacteria were stocked in 10 mL vials with 15% glycerol and kept at 4℃ for 24 h and then transferred to -20℃. The best growth was observed in the soybean medium. Therefore this medium was selected as a base medium for subsequent experiments. The bacterium was cultured in 24 similar flasks containing 25 mL soybean medium under the above mentioned conditions. Samples were drawn every 8 h for 8 days. Contents of the flask were filtered and dried in an oven at 60℃ for 3 days. Dry mass of bacteria was weighted. To confirm the production of desferrioxamine, a single colony of the bacteria was cultured on nutrient agar. After 4 days, a piece of Whatmann No 1 filter paper soaked in 1% ammonium ferric sulphate (Fe NH4(SO4)2.12 H2O] in 1% sulphuric acid was placed on the single colonies. The appearance of a brown or reddish brown halo around the colony in agar after 15 min indicated the presence of desferrioxamine B (Schupp et al 1988). The amount of desferrioxamine B in culture was measured spectrophotometrically. In this regard, the bacterium was cultured in soybean broth medium for 8day at 29℃ under shaking (150 rpm). Every 8 h, 1mL sample was withdrawn and centrifuged at 4000 rpm (4℃). The supernatant was diluted ten times with distilled water and 5 mg/mL of ammonium (ferric) sulphate in 1% sulphuric acid was added to a final concentration of 20%. The absorbance was read at 430 nm and its concentration was found by extrapolation. When the soybean was replaced with CSL and in combination with soybean in decreasing percentage of soybean, it was found that as the percentage of CSL increases, the concentration of desferrioxamine B progressively increased as shown in table 1.
|
5.3. Synthesis of Fosmidomycin Analogues as Antimalarial Agents
- Fosmidomycin has been proven to be efficient in the treatment of Plasmodium falciparum malaria by inhibiting 1-deoxy-D-xylulose–5–phosphate reductoisomerease (DXR), an enzyme of the non-mevalonate pathway, which is absent in humans. Crystal structures of fosmidomycin-bound complete quaternary complexes of PfDXR revealed that (i) an intrinsic flexibility of the PfDXR molecule accounts for an induced fit movement to accommodate the bound inhibitor in the active site and (ii) as cis arrangement of the oxygen atoms of the hydroxamate group of the bound inhibitor to the active site metal. [98] Fosmidomycin acts as a transition state analogue with the retrohydroxamic acid moiety chelating the metal ion and the phosphate group resembling the phosphate group of the substrate. The X-ray structure of DXR complexed with fosmidomycin and a divalent cation [99] revealed that the N-formyl oxygen and the N-hydroxy oxygen of fosmidomycin displace two water molecules that were bound to the divalent cation. [100] Replacement of the formyl hydrogen of fosmidomycin by a methyl group yields FR900098. The methyl group is predicted to contact the side chain of TrP212 of catalytic loop. The corresponding methyl group is also present in DOXP, which supports the view that fosmidomycin binds substrate–like and not intermediate–like. [99] This is also supported by the higher affinity of FR900098 compared to fosmidomycin. [101] The relatively high doses of fosmidomycin required for parasite clearance compared with chloroquine and the unfavorable pharmacokinetic properties prompted researchers to modify this lead. The high dose requirement is partly due to highly polar nature of fosmidomycin prompted the modifications by Haemers. [102] The N-methyl and N-ethylacetamide derivatives were synthesized starting from N-(3-bromopropyl) phthalamide (108). A Michelis-Arbusov reaction with an excess of triethyl phosphite under reflux, followed by treatment of (109) with hydrazine monohydrate yielded diethyl 3-aminopropyl phosphonate. After purification by column chromatography, amine 110 was acetylated with acetyl chloride. The resulting amine (111) was deprotonated with NaH and alkylated with iodoethane or iodomethane to give 112 and 113 respectively. Deprotection of the phosphonate esters gave the desired phosponates 114 and 115 using trimethylsilyl iodide (TMSI) or trimethylsilyl bromide (TMSBr). Generally, TMSI gave better yield & shorter reaction times.
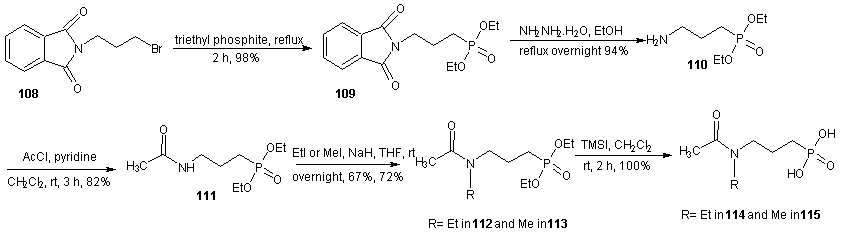 | Scheme 25 |
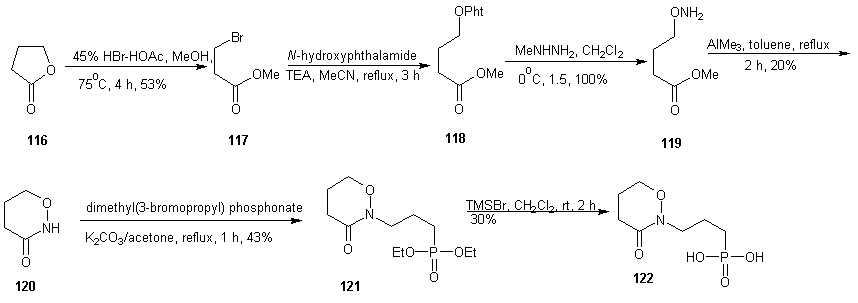 | Scheme 26 |
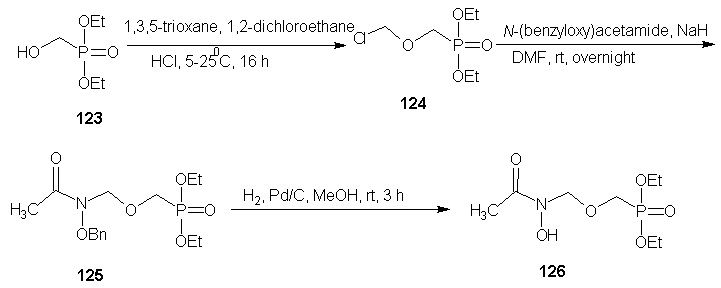 | Scheme 27 |
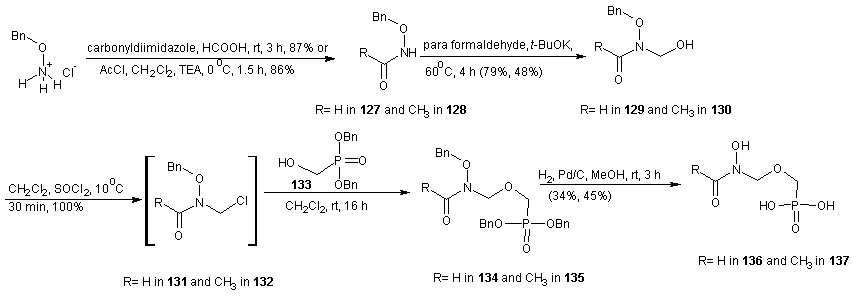 | Scheme 28 |
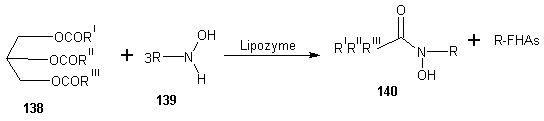
6. Enzymatic Synthesis of Fatty Acid Hydroxamic Acid Derivatives Based on Palm Kernel Oil
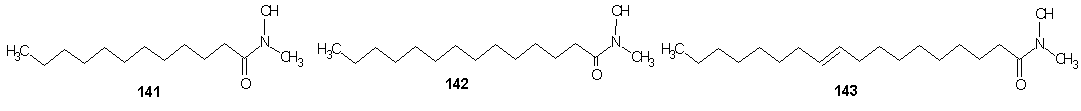
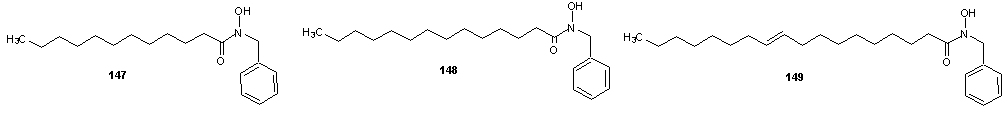
- Vaysse et al [107] reported the synthesis of fatty hydroxamic acids from fatty acids or fatty acid methyl esters and hydroxylamine using lipase-acyl transferase from Candida parapsilosis as catalyst in a biphasic lipid/aqueous medium. Recently, the synthesis of fatty hydroxamic acids (FHAs) from canola oil and hydroxylamine has been reported. [108] The reaction was carried out in biphasic medium in the presence of Lipozymes as immobilized enzymes, for optimization of the reaction, the effect of changing of reaction conditions such as types of organic solvent, pH, amount of enzyme, mole ratio of reactants and reaction time on the reaction. The synthesis of phenyl fatty hydroxamic acids (PFHAs) as the first derivatives of fatty hydroxamic acids has been reported. [109] They achieved the synthesis using phenyl hydroxyl aminolysis of canola or palm kernel oils in biphasic organic/aqueous medium using lipozyme as catalyst. The oils used had different compositions of saturated and unsaturated natural fatty acids with 12 to 22 carbon atoms in the aliphatic chain. Hence the obtained procedure is applicable to other vegetable oils. Johangirian et al [110] recently reported the synthesis of fatty hydroxamic acid derivatives using lipozyme TL 1M catalyst at biphasic medium. N-methyl fatty hydroxamic acid (MFHAs); N-isopropyl fatty hydroxamic acid (IPFHAs) and N-benzyl fatty hydroxamic acid (BFHAs) were synthesized by reaction of palm kernel oil and N-methyl hydroxylamine (N-MHA), N-isopropyl hydroxylamine (N-IPHA) and N-benzyl hydroxylamine (N-BHA) respectively.MFHAs, IPFHAs and BFHAs were synthesized from palm kernel oil according to the method earlier reported.[109] Methyl hydroxyl aminolysis, isopropyl hydroxyl aminolysis and benzyl hydroxyl aminolysis were carried out by shaking mixtures of the reactants which contained selected amounts of either N-MHA, N-IPHA or N-BHA respectively dissolve in distilled water (10 mL), palm kernel oil (710 mg, 1 mmol) dissolved in hexane (14 mL) and lipozyme TL 1M (80 mg) in a 100 mL flask sealed using Teflon film. The mixtures were shaken at 120 rpm and 39℃ in a water bath shaker for 72 h. The product was separated from the reaction mixture as follows: First, the enzyme was filtered. The filtrate was then transferred into a separation funnel for separation of aqueous phase from organic phase. The organic phase in the funnel was shaken with distilled water (10 mL) for removal of residual glycerol, and then 2M HCl solution (10 mL) was added to remove the unreacted N-MHA, N-IPHA or N-BHA. Hexane was then removed by rotatory evaporation to obtain mixture of the product (MFHAs, IPFHAs and BFHAs) and unreacted oil. Finally, the product was extracted from the unreacted oil using absolute methanol (20 mL) and then recovered by rotatory evaporation. The percentage of conversion at every experiment was calculated as follows:Conversion % = A×100/BA= amount of experimental fatty hydroxamic acid derivative obtained at every experiment.B= amount of theoretical fatty hydroxamic acid derivatives assuming all of the fatty acids in the oil were converted to fatty hydroxamic acid derivative.
 | Scheme 29 |
 | Scheme 30 |
 | Scheme 31 |
 | Scheme 32 |
7. Synthesis of Cardiovascular Hydroxamates
- Cardiovascular hydroxamate belongs to matrix metalloproteinase (MMP) inhibitors. They inactivate MMPs. [111] MMPs belong to a family of zinc dependent neutral endopeptidases. [112] These enzymes have the ability to break down connective tissue. The expression of MMP is increased is increased in various pathological conditions like inflammatory conditions, metabolic bone disease. Examples of disease are periodontitis, hepatitis, glomerulo nephritis, atherosclerosis [113] etc. Due to the role of MMP in pathological conditions inhibitors of MMP may have therapeutic potential. [113] The first generation of MMP inhibitors were based on the structure of the collagen molecule. These groups of inhibitors contain a hydroxamate group that binds the zinc atom in the active site of MMP enzyme. [114] The first MMP inhibitors that were tested in patients were ilomastat and batimastat and none showed good oral bioavailabilty. [114] Thus far, periostat is the MMP inhibitor that has been approved by the United States FDA. It is basically used for the treatment of periodontitis. Other MMP inhibitors have exhibited serious side effects during preclinical trials. This side effect has been linked to the insufficient selectivity. Most MMP inhibitors are unable to target specific MMPs connected to specific pathological conditions. [115]
7.1. Synthesis of Non-peptide Hydroxamate-based MMP Inhibitors
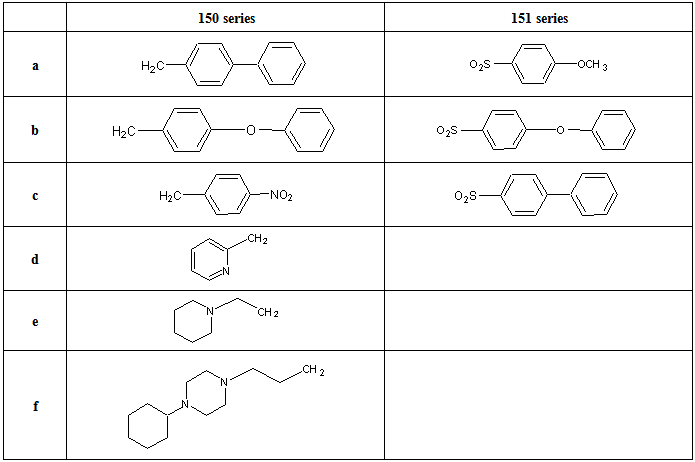
- Santos et al [116] reported the synthesis of new non-peptide hydroxamate-based MMP inhibitors by incorporating imino diacetic acid (IDA) hydroxamic acid scaffold, as mimics of truncated peptide MMP inhibitor. This new derivatives was achieved using series of alkylaryl and sulphonylaryl groups on the IDA basic scaffold. The synthesis is reported in scheme 33 and 23. The sulphonamide based IDA derivatives studied showed to be potent against deep SI’ pocket MMPs enzyme. The synthetic route for the preparation of the new compounds 150, 151 and 152 are reported in scheme 34 and 35. For the preparation of compound 150 and 151 (scheme 33), the first step involved the IDA N-coupling with the appropriate alkylaryl or sulphonylaryl halides (R1X), to give the corresponding N-substituted IDA intermediates 150a-f, 151a-c. Depending on the intermediate type, different reaction conditions have been used, namely, a homogenous phase for compounds 150a-f and Schotten-Baumann conditions for compounds 151a-c. The second step consisted of the condensation of one carboxylic group of the N-substituted IDA with hydroxylamine, upon previous activation of that group with ethyl chloroformate (ECF) in the presence of N-methyl morpholine. For the preparation of some compounds, the hydroxylamine was O-alkyl protected (alkyl = benzyl for compounds 150c1-150f1, 151a1and 151b1, and alkyl= 2,4-dimethoxybenzyl (DMB) for compounds 150c1 and 150d1), here, the alphabets with prime refers to the respective intermediates containing a carboxylic acid and an O-protected hydroxamic acids whenever it exists and the ordinary alphabet refers to the dicarboxylic acid intermediates. The DMB O-protection was selected to avoid the undesirable removal of N-alkyl substituents by catalytic hydrogenolysis with palladium, the usual method for O-benzyl de-protection.The O-DMB protected hydroxylamine was prepared by amination of the dimethoxy benzyl alcohol according to the literature. [117, 118] For these intermediates, the DMB removal was otherwise carried out in acetic media using a 5% trifluoroacetic acid in CH2Cl2. Compound 152 was prepared by first, monoamidation of the N-benzyl imino diacetic anhydride with 3-(4-phenyl piperazin-1-yl) propylamide, scheme 34, followed by condensation of the second carboxylic group with hydroxylamine in the presence of ECF and N-methylmorpholine (NMM); 3-(4-phenyl piperazin-1-yl) propylamide was prepared by standard methods [119].
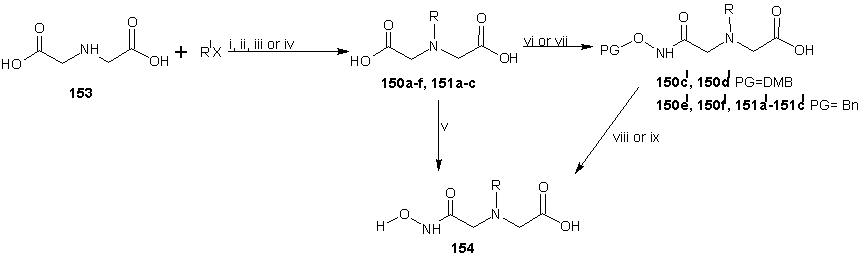 | Scheme 33 |
|
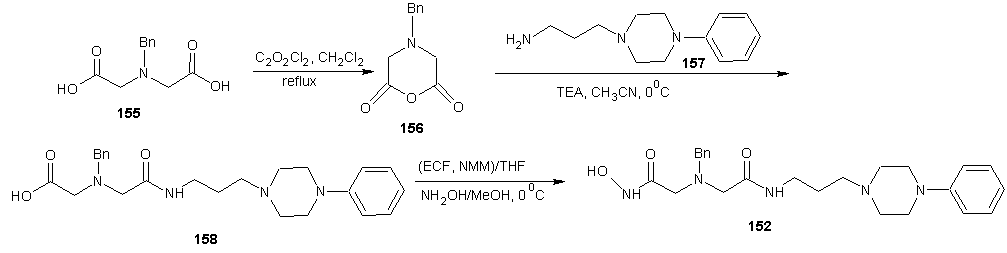 | Scheme 34 |
7.2. Synthesis of Cipemastat
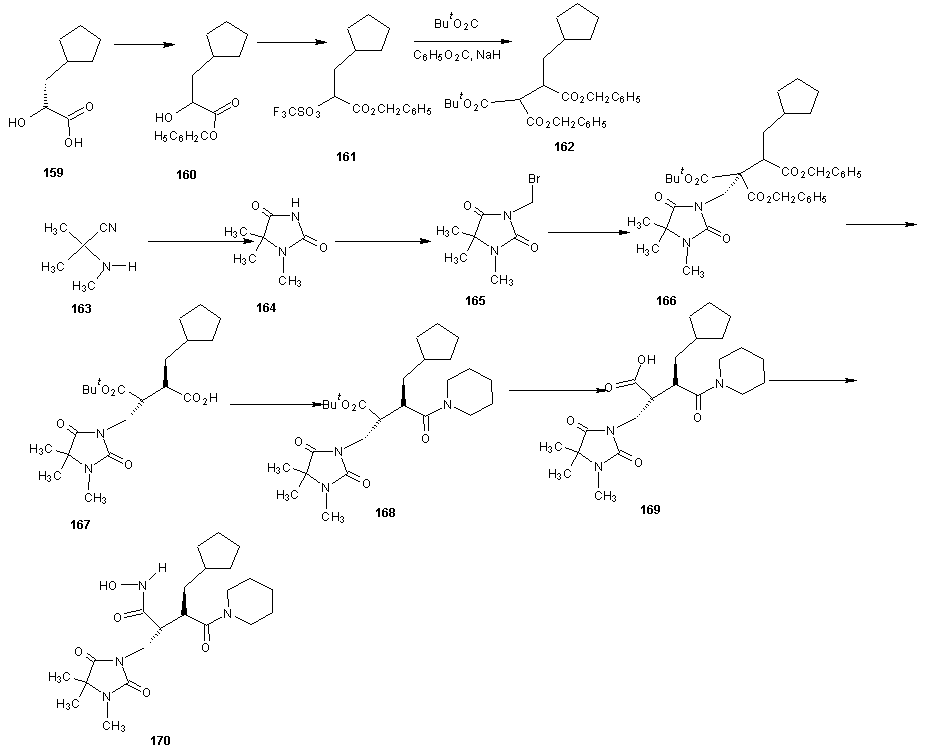 | Scheme 35 |
7.3. Synthesis of Bifunctional Metalloproteinase Inhibitors
- The preparation of all the compounds started from an amino acid or its ester derivative as reported by Marques et al. [122] In the case of compound 171, the glycine ethyl ester was used as starting material, which was coupled with the aryl sulphonyl chloride to give the secondary aryl sulphonamide (172) (in this case aryl= 4-phenoxy benzene). Compound 172 was hydrolyzed with a KOH methanolic solution to generate the carboxylic acid (173) which was coupled with O-benzyl hydroxylamine or 4-methoxy benzene sulphonyl hydrazide to afford the benzyl O-protected hydroxamic acid (174) or the aryl sulphonyl hydrazide (175) respectively. The hydroxamic acid 171 was obtained after the benzyl removal from 174 by catalytic hydrogenation with 1.5 bar H2 and 5% palladium over charcoal.
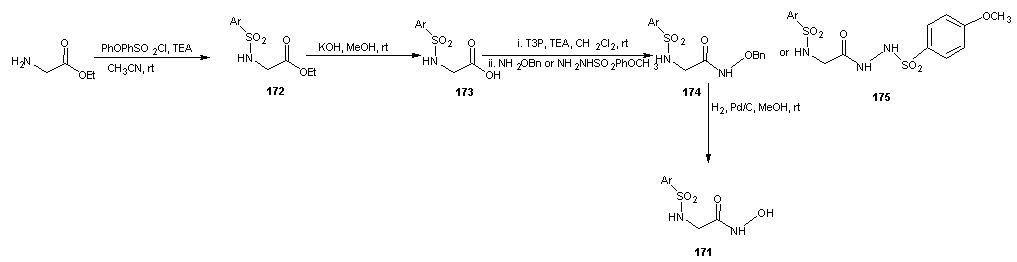 | Scheme 36 |
 | Scheme 37 |
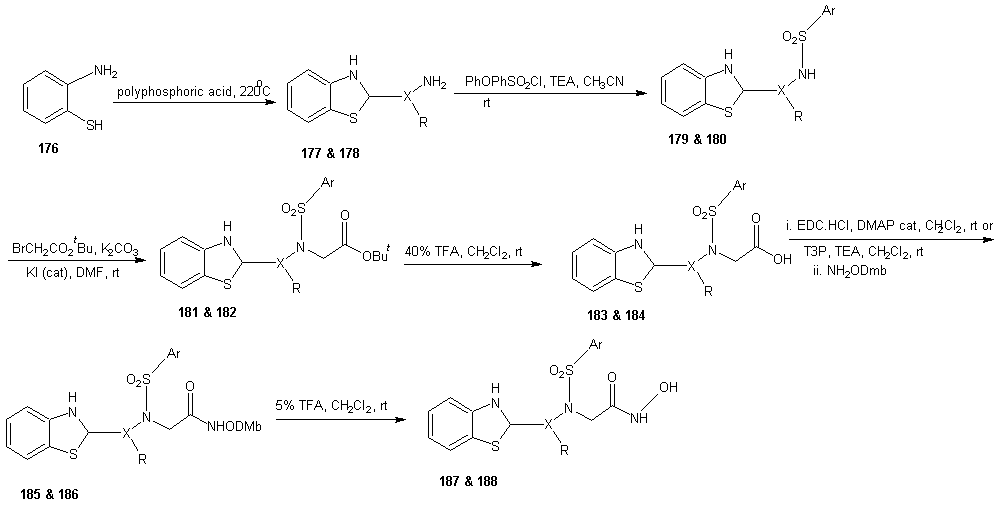
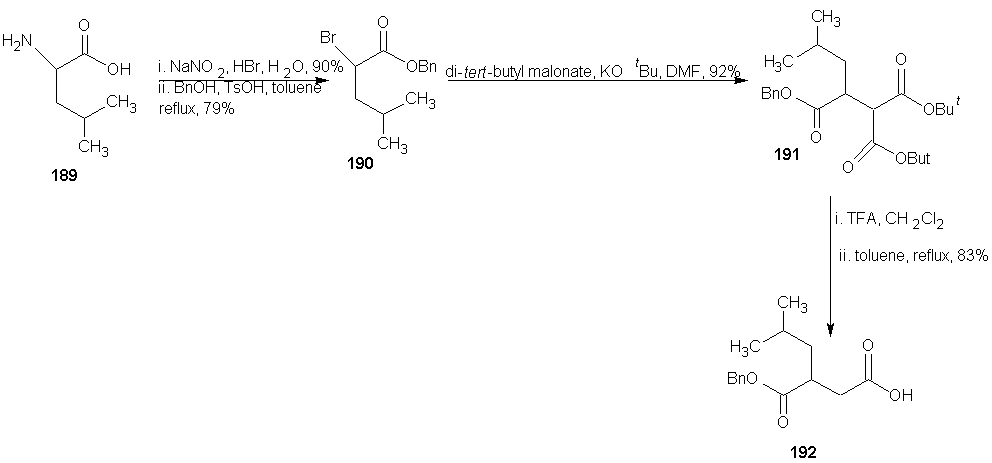
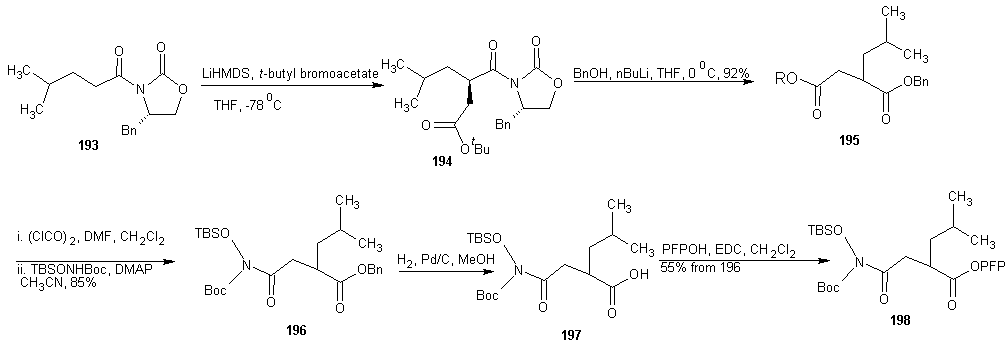
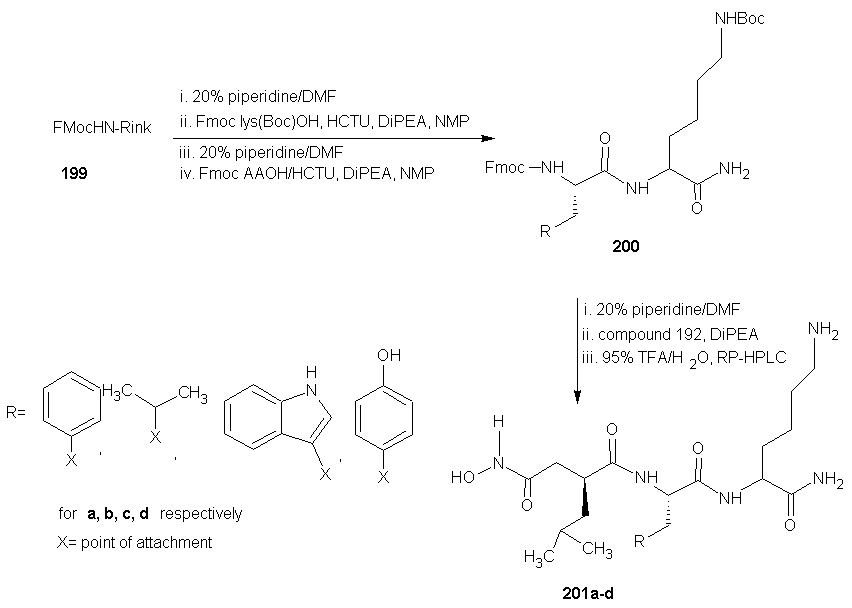
7.4. Synthesis of Peptide Hydroxamate MMP Inhibitors
- ADAMs (a disintegrin and metalloproteinase) are metalloproteinase that contain a membrane-spanning and a disintegrin (integrin binding) domain. These membrane bound enzymes are involved in membrane fusion, cytokine and growth factor shedding, cell migration, muscle development, fertilization, cellular differentiation, cell-cell interactions and cell matrix interaction. [123-125] The best known ADAM is ADAM-17 also known as tumor necrosis factor α (TNF α) converting enzyme (TACE), which was discovered based on its sheddase activity with respect to membrane-based TNF α. [126, 127] The expression of ADAMs and MMPs is regulated by transcription factors and activity is controlled by natural inhibitors, the tissue inhibitors of metalloproteinase (TIMPs). Disturbance in these regulatory mechanisms are believed to cause or be involved in a wide range of pathological diseases. These include cancer metastasis, rheumatoid arthritis and autoimmune diseases. Deregulation of ADAM expression or activity has also been linked to asthma, Alzheimer’s, bacterial lung infections and allergies of the airways. [123, 128-134]A requirement for potent MMP or ADAM inhibitors is that they contain a good zinc binding group (ZBG). This is because MMPs and ADAMs contain a Zn2+ ion in their active site, which forms a complex with the carbonyl group of the scissile amide bond. This complexation enhances the reactivity of the carbonyl towards nucleophilic attack of the water molecule that is present in the active site and also coordinated by Zn2+ ion. [129] A large number of MMP and ADAM inhibitors contains an oligopeptide sequence that is equipped with a hydroxamate moiety at either the C- or the N-terminus. Commercially available members of this type are marimastat, batimastat and TAPI-2 each displaying sub- to low-nano molar, broad MMP/ADAM inhibitory activity. The oligopeptide portion ensures recognition by the metalloproteinase by directing the substituents to the corresponding enzymes’ binding pockets. The bidentate Zn2+ chelating properties of the hydroxamic acid has the dual effect of a strong zinc coordination as well as expulsion of the nucleophilic water molecule from the active site, thereby preventing hydrolysis to occur.
 | Scheme 38 |
 | Scheme 39 |
 | Scheme 40 |
- DMAP = 4-(N,Ndimethylamino)pyridine, LHMDS = Lithium hexamethyl disilyazide.Next, they evaluated the potential of PFP-ester 198 in SPPS. Dipeptide 200 was synthesized on Rink amide resin 199 using standard SPPS protocols. After removal of the Fmoc group, several conditions for the coupling of 198 were investigated. The optional conditions proved to be shaking the resin for 2 h with 5 equivalents of 198 and 2 equivalents of DiPEA relative to the resin-bound peptide in N-Methylpyrrolidone (NMP). The resulting product was cleaved from the resin and concomitantly deprotected using 95% aqueous TFA, clearly yielding peptide 201a, in the same fashion the analogous peptides in which phenylalanine was replaced with leucine (201b, 56%), tryptophan (201c, 42%), and tyrosine (201d, 71%) were synthesized. HCTU =O-(1H-6-Chlorobenzotriazolyl)-1,1,3,3-tetramethyluroniumhexafluorophosphate, DiPEA = diisopropylethylamine, TFA = trifluoroacetic acid, NMP = N-methyl-2-pyrrolidoneAA = phenylalanine, leucine, tryptophan, tyrosine for a, b, c and d respectively.The enzyme inhibition tests using a fluorogenic substrate revealed IC50 values in the low nano molar range for the four compounds against both MMP-12 and ADAM-17. The observation that the aromatic amino acid containing compounds 201acd are more potent inhibitors than the aliphatic 201b corroborate earlier findings [129].
8. Conclusions
- Hydroxamates are physiologically important compounds. They are used as anticancer drugs given their ability to inhibit histone deacetylase (HDAC). A good number of hydroxamates have found application in cancer therapy like the trichostatin A, vorinostat, panobinostat, polynuclear diorganotin(IV) complexes etc. Some hydroxamates have been used in HIV chemotherapy like the hydroxyurea. Hydroxyurea inhibits the cellular enzyme ribonucleoside diphosphate reductase and in so doing reduces the levels of deoxy ribonucleotide. They also have synergistic effect when in combination with didanosine. In malaria chemotherapy, hydroxamates are not left out. They are potential antimalarial. They acts as chelator, their biological action has been attributed to chelation of internal iron pools and in turn, to interference with the supply of iron to different components, possibly to ribonucleotide reductase. The most pronounced antimalarial hydroxamate is desferrioxamine B has been extended to corn steep liquor in recent studies. Ilomastat, batimastat, cipemastat, periostat and marimastat are all hydroxamate compounds used in treatment of cardiovascular diseases. The cardiovascular hydroxamates inhibits matrix metalloproteinase (MMPs). They inactivate the enzymes which has the ability to break down connective tissues. Although there are many MMP inhibitors, their increased side effect has limited their applications, only periostat is approved by United States FDA. As described in the review, most hydroxamates have very lengthy synthetic route and therefore this review explored various synthetic route to hydroxamates and presents a wider literature for easy modifications of the existing hydroxamates so as to exploit their broad biological applications.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML