-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2014; 4(2): 21-25
doi:10.5923/j.ajoc.20140402.01
p-TSA Catalysted, One-Pot Synthesis and Antimicrobial Evaluation of Some Novel Fused Dipyrazolo-1,4-Dihydropyridine Derivatives
Harvinder Singh Sohal1, Manvinder Kaur2, Rajshree Khare1, Kishanpal Singh2
1Department of Chemistry, Maharishi Markandeshwar University, Mullana-133 207, Haryana, India
2Department of Chemistry, Punjabi University, Patiala-147 002, Punjab, India
Correspondence to: Harvinder Singh Sohal, Department of Chemistry, Maharishi Markandeshwar University, Mullana-133 207, Haryana, India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Some novel dipyrazolo-1,4-dihydropyridine derivatives have been synthesized via one-pot, multi-component condensation of N-phenylpyrazole, aldehyde and ammonium acetate using p-TSA as catalyst. The fused dipyrazolo-1,4-dihydropyridine molecules without using expensive starting materials and the molecules are obtained in excellent yield without using any special purification technique. The synthesized compounds were screened for their in vitro antibacterial and antifungal activity against six bacterial and four fungal species. Among these 4a, 4e and 4h exhibited significant antibacterial activity against various bacterial and fungal strains.
Keywords: p-TSA Catalysed, Multi-Component, 1,4-DHPs, MIC, Antibacterial Activity
Cite this paper: Harvinder Singh Sohal, Manvinder Kaur, Rajshree Khare, Kishanpal Singh, p-TSA Catalysted, One-Pot Synthesis and Antimicrobial Evaluation of Some Novel Fused Dipyrazolo-1,4-Dihydropyridine Derivatives, American Journal of Organic Chemistry, Vol. 4 No. 2, 2014, pp. 21-25. doi: 10.5923/j.ajoc.20140402.01.
Article Outline
1. Introduction
- The chemistry of 1,4-dihydropyridines (1,4-DHP’s) began in 1882 with Hantzsch condensation [1]. After Hantzsch, modifications of the original synthesis were developed [2]. 1,4-dihydropyridines ring system is of considerable interest because of its presence in the coenzyme, diphosphopyridine nucleotide (DPNH) [3] and found it as bio-active material. Now days many representatives have been commercialised such as nifedipine [4], felodipine [5], nicardipine [6], amlodipine [7] and many more have appeared in the market [8] in the treatment of angina and hypertension. It has been proved that their pharmaceutical action is related to binding to voltage dependent L-type of calcium channel and thus decreasing the passage of calcium ions to the cell. The result is relaxation of smooth muscle cells and lowering the blood pressure. DHPs have been explored to possess anti-tumor [8], anti-inflammatory [10], anticonvulsant activity [11], antitubercular activity [12] cerebral antischemic activity in the treatment of Alzheimer’s disease, PAF-acether antagonists [13], etc. Apart from these many fused ring system such as Etazolate (SQ-20,009, EHT-0202), ICI-190,622, Tracazolate (ICI-136,753), etc are known to show many biologicial activities. By keeping above in mind and continuation of our on hetrocyclics [14], modification to the original Hantzsch pyridines have been carried out by one-pot condensation of 5-methyl-2-phenyl-2,4-dihydro-3H -pyrazol-3-one, aldehyde and ammonium acetate in acetonitrile using p-TSA as catalyst Scheme 1.
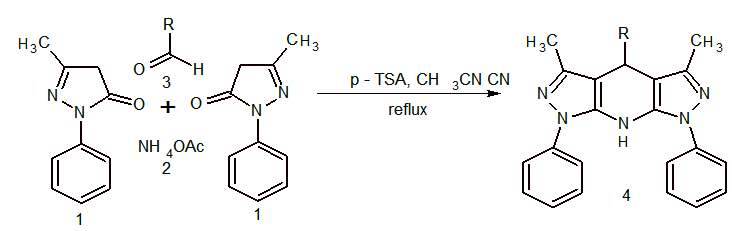 | Scheme 1. One-pot three component synthesis of Fused Dipyrazolo-1,4-dihydropyridine Derivatives |
2. Experimental
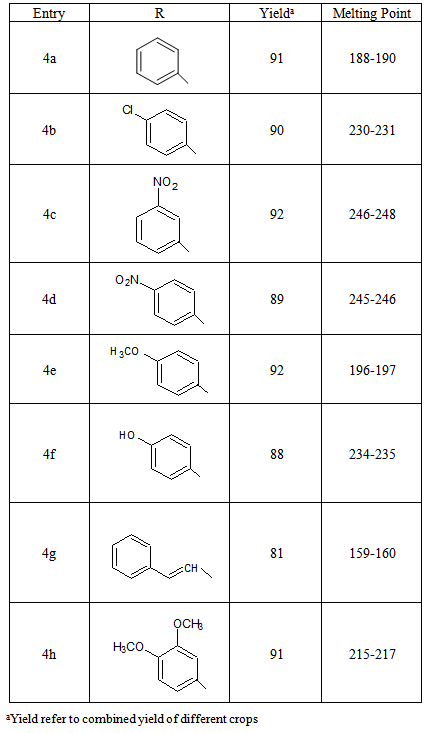
- Materials were obtained from commercial suppliers and were used without further purifications. Melting points were recorded in open end capillaries and are uncorrected. 1H NMR spectra were recorded in DMSO-d6 on a Bruker Avance II 400 MHz spectrometer; chemical shifts (delta) are reported in ppm relative to TMS as internal standard. The mass spectrum and IR spectra were recorded at LC-MS Spectrometer Model Q-ToF Micro Waters and Perkin-Elmer Spectrum II infra-red spectrophotometer, respectively. Elemental analyses (C, H, and N) were performed using a Thermo Scientific elemental analyser.3,5-dimethyl-1,4,7-triphenyl-1,4,7,8-tetrahydrodipyrazolo[3,4-b:4',3'-e]pyridine 4a: In a conical flask, Benzaldehyde (1.06mL, 10mmol), N-phenylpyrazole (3.48g, 20mmol) and ammonium acetate (1.155g, 15mmol) in CH3CN (10 mL) were heated at 90℃ for 3 hours. After the completion of reaction (vide TLC), the solvent was removed under reduced pressure. The residue obtained was washed with cold water and recrystalised from ethanol to afford 3,5-dimethyl-1, 4,7-triphenyl-1,4,7,8-tetrahydrodipyrazolo[3,4-b:4',3'-e]pyridine 4a, , in 91% yield, colourless, M.p. 188-190℃ (Entry 1, Table 2). Similarly, other aldehydes 3b-h were reacted with N-phenylpyrazole and ammonium acetate to afford various dipyrazolo-1,4-dihydropyridine derivatives 4b-h.Characterization and spectral data for some selected compounds3,5-dimethyl-1,4,7-triphenyl-1,4,7,8-tetrahydrodipyrazolo[3,4-b:4',3'-e]pyridine (4a): mp. 188–190°C; IR (KBr): υ = 3360, 3063, 3026, 2975, 1596, 1500, 1283, 1026 cm-1. 1H NMR (400 MHz, DMSO-d6): δ(ppm) = 13.84 (br s, 1H, NH), 7.12-7.92 (m, 15H, Ar-H), 5.20 (s, 1H, CH), 2.35 (s, 6H, 2xCH3). 13C NMR (100 MHz, DMSO-d6): δ(ppm) = 164.3, 159.4, 145.8, 144.9, 138.6, 133.8, 131.6, 129.9, 128.1, 125.2, 116.8, 52.6, 29.4 ppm. Anal. Calcd for C27H23N5: C, 77.67; H, 5.55; N, 16.77. Found: C, 77.66; H, 5.51; N, 16.73. MS (EI) m/z 418 (M+1, 23%).3,5-dimethyl-1,7-diphenyl-4-(4-chlorophenyl)-1,4,7,8-tetrahydrodipyrazolo[3,4-b:4',3'-e] pyridine (4b): mp. 230-231°C; IR (KBr): υ = 3369, 3053, 2977, 2920, 1600, 1501, 1296, 1024 cm-1. 1H NMR (400 MHz, DMSO-d6): δ(ppm) = 13.89 (br s, 1H, NH), 7.16-7.91 (m, 14H, Ar-H), 5.24 (s, 1H, CH), 2.38 (s, 6H, 2xCH3). 13C NMR (100 MHz, DMSO-d6): δ(ppm) = 166.4, 160.1, 146.3, 145.8, 139.8, 136.2, 133.4, 130.1, 129.2, 127.4, 117.9, 52.8, 30.1 ppm. Anal. Calcd. for C27H22N5Cl: C, 71.75; H, 4.91; N, 15.50. Found: C, 71.72; H, 4.91; N, 15.47; MS (EI) m/z 453 (M+1, 18%).3,5-dimethyl-1,7-diphenyl-4-(3-nitrophenyl)-1,4,7,8-tetrahydrodipyrazolo[3,4-b:4',3'-e] pyridine (4c): mp. 246-248°C; IR (KBr): υ = 3436, 3075, 2920, 1601, 1527, 1285, 1021 cm-1. 1H NMR (400 MHz, DMSO-d6): δ(ppm) = 13.92 (br s, 1H, NH), 7.26-8.10 (m, 14H, Ar-H), 5.31 (s, 1H, CH), 2.44 (s, 6H, 2xCH3). 13C NMR (100 MHz, DMSO-d6): δ(ppm) = 167.2, 161.2, 158.3, 155.5, 149.3, 146.4, 142.9, 140.2, 138.6, 137.5, 133.2, 129.2, 121.0, 55.3, 32.6 ppm. Anal. Calcd. for C27H22N6O2: C, 70.12; H, 4.79; N, 18.17. Found C, 70.10; H, 4.78; N, 18.17. MS (EI) m/z 463 (M+1, 20%). 3,5-dimethyl-1,7-diphenyl-4-(4-methoxyphenyl)-1,4,7,8-tetrahydrodipyrazolo[3,4-b:4',3'-e] pyridine (4e): mp. 196-197°C; IR (KBr): υ = 3412, 2919, 2839, 1600, 1507, 1180, 1032 cm-1. 1H NMR (400 MHz, DMSO-d6): δ(ppm) = 13.81 (br s, 1H, NH); 7.06-7.82 (m, 14H, Ar-H), 5.18 (s, 1H, CH), 3.87 (s, 3H, OCH3), 2.31 (s, 6H, 2xCH3). 13C NMR (100 MHz, DMSO-d6): δ(ppm) = 163.4,158.2, 144.3, 143.7, 136.8, 132.9, 130.2, 128.6, 125.5, 124.3, 113.2, 59.6, 47.3, 28.2 ppm. Anal. Calcd. for C28H25N5O: C, 75.15; H, 5.63; N, 15.65. Found C, 75.14; H, 5.61; N, 15.63. MS (EI) m/z 448 (M+1, 16%).3,5-dimethyl-1,7-diphenyl-4-(3,4-dimethoxyphenyl)-1,4,7,8-tetrahydrodipyrazolo[3,4-b:4',3'-e] pyridine (4h): mp. 215-217°C; IR (KBr): υ = 3409, 2906, 2830, 1601, 1505, 1190, 1022 cm-1. 1H NMR (400 MHz, DMSO-d6): δ(ppm) = 13.71 (br s, 1H, NH), 6.92-7.70 (m, 13H, Ar-H), 5.09 (s, 1H, CH), 3.92 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 2.29 (s, 6H, 2xCH3). 13C NMR (100 MHz, DMSO-d6): δ(ppm) = 162.3, 155.8, 152.1, 149.3, 142.1, 140.2, 139.8, 138.9, 135.2, 133.4, 129.6, 126.2, 119.7, 62.5, 51.4, 28.2 ppm. Anal. Calcd. for C29H27N5O2: C, 72.94; H, 5.70; N, 14.66. Found: C, 72.90; H, 5.67; N, 14.65. MS (EI) m/z 478 (M+1, 13%).
3. Result and Discussion
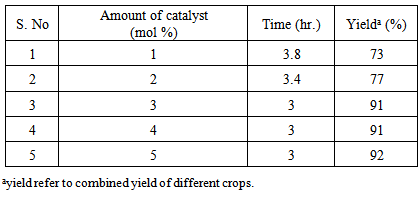
- The reactions of benzaldehyde, N-phenylpyrazole and ammonium acetate were refluxed in the varying amount of p-TSA as catalyst in acetonitrile. The reaction conditions were optimised and it was found that 3 mol % (50 mg) p-TSA is sufficient to catalyse the reaction. It was found that decrease in the amount of catalyst will decrease the reaction yield and increase the reaction time but on increasing the amount of catalyst will not much affect both reaction time and yield.
|
|
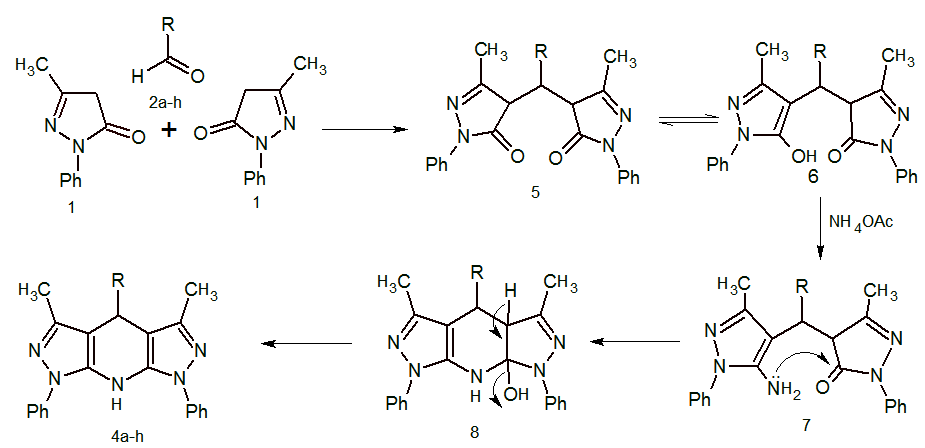 | Scheme 2. Mechanism for the synthesis of Fused Dipyrazolo-1,4-dihydropyridine Derivative |
4. Conclusions
- The present work provides an excellent route for the synthesis of novel fused dipyrazolo-1,4-dihydropyridine using readily available starting material. In addition, this protocol is easy to reproduce and do not require any tedious workup conditions. The targeted molecules are obtained in excellent yield without any side product and all the compounds evaluated against various bacterial and fungal strains. Compounds 4a, 4e and 4h exhibited significant antibacterial activity against various bacterial and fungal strains comparable to standard drug. The importance of such work lies in the possibility that the new compounds might be more efficacious drugs against bacteria for which a thorough investigation regarding the structure–activity relationship, toxicity and their biological effects could be helpful in designing more potent antibacterial agents for therapeutic use.
5. Antibacterial Activity
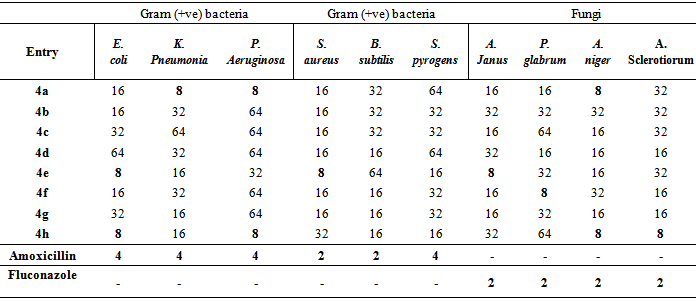
- Synthesized compounds 4a-h were screened for their in vitro antibacterial and antifungal activity against six bacterial and four fungal species namely Klubsellia pneumonia (MTCC 3384), Pseudomonas aeruginosa (MTCC 424), Escherichia coli (MTCC 443), Straphylococcus aureus (MTCC 96), Bacillius subtilis (MTCC 441), Streptoccus pyrogens (MTCC 442) and Aspergillius janus (MTCC 2751), Pencillium glabrum (MTCC 4951), Aspergillus sclerotiorum (MTCC 1008), Aspergillus niger (MTCC 281) respectively. Amoxicillin and fluconazole were used as the standard drug as positive control while the DMSO was used as negative control.The minimum inhibitory concentrations of the newly prepared compounds (4a–4h) were determined by using Serial tube dilution method at the concentration of 128, 64, 32, 16, 8, 4, 2 and 1 lg/ml against above said microorganisms. The bacterial and fungi strains susceptibility to the studied compounds was determined by the appearance of turbidity after 24 h of incubation at 37℃ and 72 h of incubation at 28℃, respectively. The observed MIC values (µg/ml) for the compounds 4a-4h are represented in table 3.It is evident from Table 3 that the compounds 4a, 4e and 4h show significant activities against the most of bacterial and fungal strains at (MIC-8 µg/ml). The compound 4a showed significant activity (MIC-8 µg/ml) against the strains, K. pneumonia, P. aeruginosa, S. aureus, B. subtilis (bacterial strains) and A. niger (fungi strains) while 4e displayed activity of same order against E. coli, S. aureus and A. janu. Further 4h showed significant activity (MIC-8 µg/ml) against E. coli, P. aeruginosa (bacterial strains) and A. niger, A. sclerotiorum (fungi strains).
|
ACKNOWLEDGEMENTS
- The authors thank Maharishi Markandeshwar University, Mullana-133 207, Haryana, India for the financial support and Harvinder Singh Sohal also thank Mr. Vikas Pahwa for the liberal support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML