-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2013; 3(1): 1-8
doi:10.5923/j.ajoc.20130301.01
Synthesis and Characterization of New Schiff Bases Heterocyclic Compounds and Their N-Acyl, Thiourea and Imidazole Derived from D-Erythroascorbic Acid
Muna S. Al-Rawi , Jumbad H. Tomma , Abdul-Jabber A. Mukhlus , Ammar H. Al-Dujaili
Department of Chemistry, College of Education, Ibn Al-Haytham, University of Baghdad, Baghdad, Iraq
Correspondence to: Ammar H. Al-Dujaili , Department of Chemistry, College of Education, Ibn Al-Haytham, University of Baghdad, Baghdad, Iraq.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The new Schiff bases derived from D-erythroascorbic acid containing heterocyclic unit were synthesized by condensation of D-erythroascorbic acid with aromatic amine (containing 1,3,4-oxadiazole or 1,3,4-thiadiazole unit) in dry benzene using glacial acetic acid as a catalyst. D-erythroascorbic acid[IV] was synthesized by four steps (Scheme 1), while the primary aromatic amine which is containing 1,3,4-oxadiazole[VII] or 1,3,4-thiadiazole[XII] synthesized by the reaction of 4-methoxybenzoyle- hydrazine[VI] with 4-aminobenzoic acid or by the reaction tuloic acid with thiosemicarbazide, respectively in the presence of POCl3. The new imidazole derivatives were synthesized by three-steps reactions starting with corresponding Schiff bases[VIII ] or[XIII ]. N-acyl compounds[IX]a,b and[XIV]a,b were synthesized by addition reaction of acid chloride to imine group of Schiff bases in dry benzene. The second step include reaction of thiourea with N-acyl derivatives in Na2CO3 medium to yield N-thiourea compounds[X]a,b and[XV]a,b. The third step involves cyclization reaction of N-thiourea derivatives with benzoin in DMF to result new imidazole derivatives[XI]a,b and[XVI]a,b. The structure of the synthesized compounds have been characterized by their melting points, elemental analysis and by their spectral data; FTIR, UV-Vis, mass, 1HNMR, and 13CNMR spectroscopy. All the synthesized compounds have been screened for their antibacterial activities. They exhibited good antibacterial activity against Escherichia coli (G-) and Staphylococus aureus (G+).
Keywords: Schiff bases, L-Ascorbic acid, Imidazole, 1,3,4-oxadiazole, 1,3,4-thiadiazole
Cite this paper: Muna S. Al-Rawi , Jumbad H. Tomma , Abdul-Jabber A. Mukhlus , Ammar H. Al-Dujaili , Synthesis and Characterization of New Schiff Bases Heterocyclic Compounds and Their N-Acyl, Thiourea and Imidazole Derived from D-Erythroascorbic Acid, American Journal of Organic Chemistry, Vol. 3 No. 1, 2013, pp. 1-8. doi: 10.5923/j.ajoc.20130301.01.
Article Outline
1. Introduction
- D-erythroascorbic acid derivatives shows antitumor and antiviral activities[1,2]. Schiff bases are used as substrates in the preparation of a large of bioactive and industrial compounds via ring closure, cycloaddiation and replacement reactions. In addition, Schiff bases are well known to have biological activities[3-5]. The thioureas have been shown to possess antifungal properties, antimicrobial[6] and potential antiviral agents, [7,8] also these compounds have been widely used in enantioselective synthesis such as in Mannich reaction, Michael addition and so on[9].Imidazoles like all azoles are five membered ring systems, [10] occurs in purine nucleus and in histidine[11,12]. The imidazoles derivatives have useful applications in such areas as medical fields: anti-histamine drugs, antifungi,[13] antitumor,[14] anti-inflammatory activity andanticonvulsant activity[15]. To the best of our knowledge that the synthesis of imidazoles derivatives derived from D-erythroscorbic acid are not reported in literature. Thus, we report herein the synthesis, characterization and antibacrial activity of new derivatives of imidazole containing D-erythroscorbic acid
2. Experimental
2.1. Materials
- All the chemicals were supplied from Aldrich-Sigma Chemicals Co. and used as received. FTIR spectra were recorded using potassium bromide discs on a 8400s Shimadzu spectrophotometer. The 1H NMR spectra were recorded on Bruker AMX-300 spectrometer at 300 MHz, using deutrated chloroform or DMSO as solvent with TMS as an internal standard. Elemental analysis (C,H,N) were carried out using a Perkin-Elmer model 2400 instrument. Uncorrected melting points (uncorrected) were determined by using hot-stage Gallen Kamp melting point apparatus. UV-vis spectra of solutions were performed on CECL 7200 England Spectrophotometer using CHCl3 as a solvent. Mass spectra were recorded on IEOL JMS-7 high resolution instrument. 13C-NMR spectra of the compounds were recorded on a varian Mercury plus 100 MHZ spectrometer.
2.2. Synthesis Procedures
- All compounds were synthesized according to Schemes 1 to 3, and the following procedures. All known compounds gave acceptable elemental analysis, FTIR and NMR spectra that matched data reported in the cited references.Compounds[I] to[IV] were synthesised following the Scheme 1.
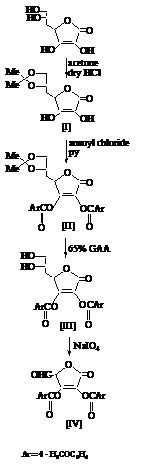 | Scheme 1. The synthesis rout for Compounds[I] to[IV] |
2.2.1. Preparation of 5,6-O-isopropylidene-L-ascorbic acid[I]
- This compound was prepared from the reaction of L-ascorbic acid with Acetone in a acidic media, following Salomon method[16].
2.2.2. Preparation of 2,3-O-dianisoyl-5,6-O-isopropylidene- L- ascorbic acid[II]
- To a cold solution of[I] (10 g, 46 mmol) in pyridine (50 mL), anisoyl chloride was added (22.3 g, 129 mmol) with stirring for 2 hrs, then kept in dark place at room temperature for 24 hrs. The mixture was poured into ice-water the oil layer was extracted with (150 mL) chloroform, washed with water and dried over anhydrous magnesium sulfate. Filtered and the solvent evaporated, purified from chloroform: petroleum ether (1:5) to give[II] (76.5%) as a pale yellow solid, m.p (102 -104℃), Rf = 0.80 (benzene/heptanol 5:5).
2.2.3. Preparation of 2,3-O-dianisoyl-L-ascorbic acid[III]
- Compound[II] (10 g, 24 mmol) was dissolved in a mixture of (65%) acetic acid (30 mL), absolute methanol (10 mL) and stirred for 48 hrs at room temperature. To the resulting solution a benzene (40mL) was added and evaporated to yield[III],[17] (78%) as a white crystals, m.p (130-132℃), Rf = 0.42 (benzene/methanol 4:6).
2.2.4. Preparation of pentulosono-lacton-2,3-ene-dianisoate [IV]
- To a stirred solution of sodium periodate (5.6 g) in distilled water ( 60 mL) at (0℃), a solution of[III] (10 g, 26 mmol) in absolute ethanol (60 mL) was added dropwise. After stirring15 min, ethylene glycol (0.5 mL) was added and stirring for one hour. The mixture was extracted with ethyl acetate (3x50 mL)[17]. The extracts dried over anhydrouse MgSO4, filtered and the solvent evaporated, the residue recrystallized from benzene to yield[IV] (45%) as a white crystals, m.p (156-158℃), Rf=0.70 (benzene / methanol 6:4).Compounds[V] to[XI] were synthesised following the Scheme 2.
2.2.5. Preparation of Methyl 4-Methoxybenzoate[V]
- This compound was prepared following the procedure described by Vog el[18], m.p.(49-51℃).
2.2.6. Preparation of Methoxy Benzoyl Hydrazine[VI]
- This compound was prepared following the procedure described by Smith[19]. m.p.(135-137℃).
2.2.7. Preparation of 2-(4′-aminophenyl)-5-(4′′-methoxy - phenyl) -1,3,4-oxadiazole[VII]
- A mixture of 4-methoxybenzoyl hydrazine (10 mmol), 4-aminobenzoic acid (10 mmol) and phosphorus oxy chloride (5 mL) was refluxed for 7 hrs. The cold reaction mixture was poured into ice-water and made basic by adding sodium bicarbonate solution. The formed solid was filtered, dried and purified. Yield: 89%; m.p. = 196 ℃ (ethanol);[20]. IR(KBr)
 cm-1: 3350-3200 (NH2), 2935-2841(C-H), 1610 (C=N), 1245 ( sym. C-O-C), 1070 (asym. C-O-C); 1H NMR (TMS) δ ppm: 6.70-8.01 (m, 8 H, ArH ), 3.90 (s, 3 H, OCH3), 1.60-1.75 (s, 2H, NH2) 13C NMR (CDCl3,75 MHz): δ 162.2 ( C- NH2), 114.5-128.6 (aromatic and olefinic carbons), 101.8 (C-1), 80.0, 79.1, (O-C=N), 55.5 (OCH3); Anal. Calcd. for C15H13O2N3 (267): C, 67.42; H, 4.87; N, 15.73. Found: C, 67.89; H, 5.44; N, 15.49.
cm-1: 3350-3200 (NH2), 2935-2841(C-H), 1610 (C=N), 1245 ( sym. C-O-C), 1070 (asym. C-O-C); 1H NMR (TMS) δ ppm: 6.70-8.01 (m, 8 H, ArH ), 3.90 (s, 3 H, OCH3), 1.60-1.75 (s, 2H, NH2) 13C NMR (CDCl3,75 MHz): δ 162.2 ( C- NH2), 114.5-128.6 (aromatic and olefinic carbons), 101.8 (C-1), 80.0, 79.1, (O-C=N), 55.5 (OCH3); Anal. Calcd. for C15H13O2N3 (267): C, 67.42; H, 4.87; N, 15.73. Found: C, 67.89; H, 5.44; N, 15.49.2.2.8. Preparation of Schiff bases[VIII]
- A mixture of new primary amine compounds[VII] (10 mmol), aldehyde[IV] (10 mmol), dry benzene (15 mL) and 2 drops of glacial acetic acid was refluxed for 6hrs. The solvent was evaporated under vacuum and the residue crystallized from chloroform.
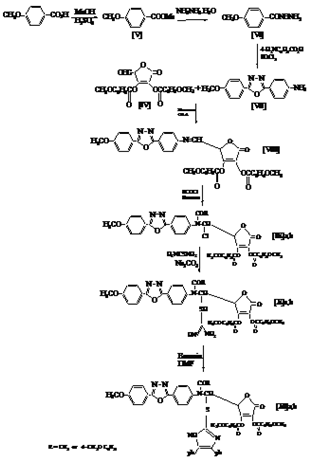 | Scheme 2. The synthesis rout for Compounds[V] to[XI] |
2.2.9. Compound[VIII]
- Yield: 75%; m.p. = 178-180 ℃(chloroform); IR(KBr)
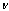 cm-1: 1635 (C=N), 1685 (C=C), 1770 (C=O); 1H NMR (TMS) δ ppm: 10.3 (s, 1H, CH=N ), 7.10-8.20 (m, 16 H, ArH ), 3.85 (s, 9 H, OCH3), 3.99 (s, 1H, lacton); Anal. Calcd. for C36H27O10N3 (661): C, 65.36; H, 4.08; N, 6.35. Found: C, 65.18; H, 4.28; N, 6.45.
cm-1: 1635 (C=N), 1685 (C=C), 1770 (C=O); 1H NMR (TMS) δ ppm: 10.3 (s, 1H, CH=N ), 7.10-8.20 (m, 16 H, ArH ), 3.85 (s, 9 H, OCH3), 3.99 (s, 1H, lacton); Anal. Calcd. for C36H27O10N3 (661): C, 65.36; H, 4.08; N, 6.35. Found: C, 65.18; H, 4.28; N, 6.45.2.2.10. Preparation of N-(acetyl or anisoyl) Derivatives[IX] a,b
- To a stirred solution of Schiff bases[VIII] (10 mmol) in 10 mL dry benzene was added dropwise acetyl or anisoyl chloride (12 mmol) after cooling , the reaction mixture was refluxed for 1 hour. The solvent was evaporated and the residue was washed with water for many times and recrystallized from petroleum ether.
2.2.11. Compound[IX]a
- Yield: 60%; m.p. = 122-124 ℃(petroleum ether); IR(KBr)
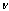 cm-1: 2939-2843 (C-H), 1767(C=O lactone), 1687(C=C), 1708 (C=O amide), 748 (C-Cl); 1H NMR (TMS) δ ppm: 7.30 (s, 1H, CH=N ), 7.00-8.04 (m, 16 H, ArH ), 3.85 (s, 9 H, OCH3), 2.88 (s, 3 H, CH3), 3.98 (s, 1H, lacton); Anal. Calcd. for C38H30O11N3Cl (739.5): C, 61.66; H, 4.06; N, 5.68. Found: C, 61.53; H, 4.31; N, 5.79.
cm-1: 2939-2843 (C-H), 1767(C=O lactone), 1687(C=C), 1708 (C=O amide), 748 (C-Cl); 1H NMR (TMS) δ ppm: 7.30 (s, 1H, CH=N ), 7.00-8.04 (m, 16 H, ArH ), 3.85 (s, 9 H, OCH3), 2.88 (s, 3 H, CH3), 3.98 (s, 1H, lacton); Anal. Calcd. for C38H30O11N3Cl (739.5): C, 61.66; H, 4.06; N, 5.68. Found: C, 61.53; H, 4.31; N, 5.79.2.2.12. Compound[IX]b
- Yield: 65%; m.p. = 146-148℃ (petroleum ether); IR(KBr)
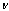 cm-1: 2935-2839 (C-H), 1771(C=O lactone), 1686(C=C), 1700(C=O amide), 767 (C-Cl); Anal. Calcd. for C44H34O12N3Cl (831.5): C, 63.50; H, 4.09; N, 5.05. Found: C, 63.37; H, 4.28; N, 5.19.
cm-1: 2935-2839 (C-H), 1771(C=O lactone), 1686(C=C), 1700(C=O amide), 767 (C-Cl); Anal. Calcd. for C44H34O12N3Cl (831.5): C, 63.50; H, 4.09; N, 5.05. Found: C, 63.37; H, 4.28; N, 5.19.2.2.13. Preparation of N-(acetyl or anisoyl )Thioureas Derivatives[X]a,b
- A mixture of compound[IX]a,b (10 mmol), thiourea (10 mmol), anhydrous sodium carbonate (10 mmol), 20 mL analar acetone was refluxed for 4 hrs with stirring. The reaction mixture was cooled and poured into ice water. The product was filtered off and recrystalized from ethyl acetate to give colored compounds[X]a,b.
2.2.14. Compound[X]a
- Yield: 60%; m.p. = 240-242 ℃(petroleum ether); IR(KBr)
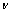 cm-1: 3393-3200 (NH2), 1700 (C=C), 1630 (C=N), 840 (C-S); 1H NMR (TMS) δ ppm: 7.88 (s, 1H, NH ), 6.95-7.08 (m, 12 H, ArH ), 3.44-3.49 (s, 2 H, NH2 ), 3.79 (s, 6 H, OCH3), 2.07 (s, 3 H, CH3), 2.72 (s, 3 H, COCH3), 3.88 (s, 1H, lacton); Anal. Calcd. for C39H33O11N5S (779): C, 60.08; H, 4.24; N, 8.99. Found: C, 59.89; H, 4.23; N, 8.74.
cm-1: 3393-3200 (NH2), 1700 (C=C), 1630 (C=N), 840 (C-S); 1H NMR (TMS) δ ppm: 7.88 (s, 1H, NH ), 6.95-7.08 (m, 12 H, ArH ), 3.44-3.49 (s, 2 H, NH2 ), 3.79 (s, 6 H, OCH3), 2.07 (s, 3 H, CH3), 2.72 (s, 3 H, COCH3), 3.88 (s, 1H, lacton); Anal. Calcd. for C39H33O11N5S (779): C, 60.08; H, 4.24; N, 8.99. Found: C, 59.89; H, 4.23; N, 8.74.2.2.15. Compound[X]b
- Yield: 45%; m.p.=213-215℃ (petroleum ether); IR(KBr)
 cm-1: 3375-3232(NH2), 2924-2850 (C-H), 1721(C=C), 1640(C=N), 841 (C-S); Anal. Calcd. for C45H37O12N5S (871): C, 62.00; H, 4.25; N, 8.04. Found: C, 61.98; H, 4.21, N, 8.17.
cm-1: 3375-3232(NH2), 2924-2850 (C-H), 1721(C=C), 1640(C=N), 841 (C-S); Anal. Calcd. for C45H37O12N5S (871): C, 62.00; H, 4.25; N, 8.04. Found: C, 61.98; H, 4.21, N, 8.17.2.2.16. Preparation of Diphenylimidazoles Derivatives[XI] a,b
- To a stirred solution of compound[X] (10 mmol) in dry DMF (10 mL), the benzoin (10 mmol) was added. The reaction mixture was refluxed for 5 hrs. After cooling, drops of water were added with stirring until a precipitate separated out. The precipitate was filtered, dried and recrystalized from petroleum ether.
2.2.17. Compound[XI]a
- Yield: 50%; m.p. = 112-114 ℃ (petroleum ether); IR(KBr)
 cm-1: 3280(NH2),, 2926-2854(C-H), 1685(C=C), 1591(C=N), 875 (C-S); Anal. Calcd. for C53H41O11N5S (855): C, 74.39; H, 4.80; N, 8.19. Found: C, 74.53; H, 4.71; N, 8.29.
cm-1: 3280(NH2),, 2926-2854(C-H), 1685(C=C), 1591(C=N), 875 (C-S); Anal. Calcd. for C53H41O11N5S (855): C, 74.39; H, 4.80; N, 8.19. Found: C, 74.53; H, 4.71; N, 8.29.2.2.18. Compound[XI]b
- Yield: 40%; m.p. = 120 ℃(petroleum ether); IR(KBr)
 cm-1: 3236(NH2), 2924-2846(C-H), 1685(C=C), 1597(C=N), 841 (C-S); 1H NMR (TMS) δ ppm: 8.06 (s, 1H, NH ), 7.07-7.20 (m, 10 H, ph ), 7.66-7.93 (s, 20 H, ArH ), 3.86 (s, 12 H, OCH3), 3.74 (s, 1 H, C4 lacton), 7.35 (s, 1 H, C5 lacton), 3.88 (s, 1H, lacton); Anal. Calcd. for C59H45O12N5S (1047): C, 67.62; H, 4.30; N, 6.69. Found: C, 67.77; H, 4.17; N, 6.79.Compounds[XII] to[XVI] were synthesised following the Scheme 3.
cm-1: 3236(NH2), 2924-2846(C-H), 1685(C=C), 1597(C=N), 841 (C-S); 1H NMR (TMS) δ ppm: 8.06 (s, 1H, NH ), 7.07-7.20 (m, 10 H, ph ), 7.66-7.93 (s, 20 H, ArH ), 3.86 (s, 12 H, OCH3), 3.74 (s, 1 H, C4 lacton), 7.35 (s, 1 H, C5 lacton), 3.88 (s, 1H, lacton); Anal. Calcd. for C59H45O12N5S (1047): C, 67.62; H, 4.30; N, 6.69. Found: C, 67.77; H, 4.17; N, 6.79.Compounds[XII] to[XVI] were synthesised following the Scheme 3.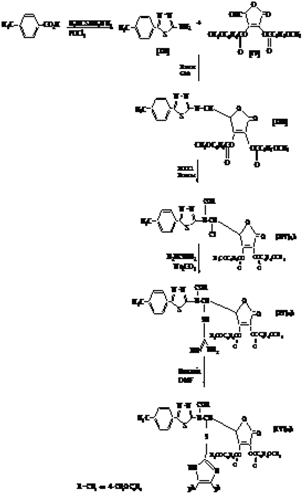 | Scheme 3. The synthesis rout for Compounds[XII] to[XVI] |
2.2.19. preparation of 2-amino-5-(4`-tolyl)-1,3,4-thiadia- zole[XII ]
- A mixture of tuloic acid (10 mmol), thiosemicarbazide (10 mmol), and phosphorus oxy chloride (5 mL) was refluxed gently for 6 hrs. After cooling, ice water (50 mL) was added in portions with stirring. The yellow precipitate was filtered, washed with water , dried and crystallized from ethanol yield(68%), m.p.(246-248℃)[21].
2.2.20. Preparation of Schiff bases [XIII]
- A mixture of new primary amine compounds[VIII] (10 mmol), aldehyde[IV] (10 mmol), dry benzene (15 mL) and 2 drops of glacial acetic acid was refluxed for 6hrs. The solvent was evaporated under vacuum and the residue crystallized from chloroform.
2.2.21. Compound[XIII]
- Yield: 60%; m.p. = 162-164 ℃(chloroform); IR(KBr)
 cm-1: 1636(C=N), 1685 (C=C), 1771(C=O); 1H NMR (TMS) δ ppm: 8.9 (s, 1H, CH=N ), 6.99-7.90 (m, 12 H, ArH ), 3.81 (s, 6 H, OCH3), 2.35 (s, 3 H, CH3), 3.98 (s, 1H, lacton); Anal. Calcd. for C30H23O8N3S (585): C, 61.45; H, 3.93; N, 7.18. Found: C, 61.44; H, 4.13; N, 7.91.
cm-1: 1636(C=N), 1685 (C=C), 1771(C=O); 1H NMR (TMS) δ ppm: 8.9 (s, 1H, CH=N ), 6.99-7.90 (m, 12 H, ArH ), 3.81 (s, 6 H, OCH3), 2.35 (s, 3 H, CH3), 3.98 (s, 1H, lacton); Anal. Calcd. for C30H23O8N3S (585): C, 61.45; H, 3.93; N, 7.18. Found: C, 61.44; H, 4.13; N, 7.91.2.2.22. Preparation of N-(acetyl or Anisoyl) Derivatives [XIV] a, b
- To a stirred solution of Schiff bases[XIII] (10 mmol) in 10 mL dry benzene was added dropwise acetyl or anisoyl chloride (12 mmol) after cooling , the reaction mixture was refluxed for 1 hour. The solvent was evaporated and the residue was washed with water for many times and recrystallized from petroleum ether.
2.2.23. Compound[XIV]a
- Yield: 65%; m.p. = 146-148 ℃(petroleum ether); IR(KBr)
 cm-1: 2900-2843(C-H), 177(C=O lactone)0, 1685(C=C), 1708(C=O amide), 1685(C=C), 772 (C-Cl); Anal. Calcd. for C32H26O9N3SCl (663.5): C, 57.87; H, 3.92; N, 6.33. Found: C, 57.73; H, 4.10; N, 6.45.
cm-1: 2900-2843(C-H), 177(C=O lactone)0, 1685(C=C), 1708(C=O amide), 1685(C=C), 772 (C-Cl); Anal. Calcd. for C32H26O9N3SCl (663.5): C, 57.87; H, 3.92; N, 6.33. Found: C, 57.73; H, 4.10; N, 6.45.2.2.24. Compound[XIV]b
- Yield: 50%; m.p. = 179-180℃ (petroleum ether); IR(KBr)
 cm-1: 2925-2850(C-H), 1775(C=O lactone), 1685(C=C), 1720(C=O amide), 1687(C=C), 772 (C-Cl); Anal. Calcd. for C38H30O10N3SCl (655.5): C, 69.57; H, 4.58; N, 6.41. Found: C, 69.43; H, 4.54; N, 6.27 .
cm-1: 2925-2850(C-H), 1775(C=O lactone), 1685(C=C), 1720(C=O amide), 1687(C=C), 772 (C-Cl); Anal. Calcd. for C38H30O10N3SCl (655.5): C, 69.57; H, 4.58; N, 6.41. Found: C, 69.43; H, 4.54; N, 6.27 .2.2.25. Preparation of N-(acetyl or anisoyl )Thioureas Derivatives[XV] a,b
- A mixture of compound[XIV]a,b (10 mmol), thiourea (10 mmol), anhydrous sodium carbonate (10 mmol), 20 mL analar acetone was refluxed for 4 hrs with stirring. The reaction mixture was cooled and poured into ice water. The product was filtered off and recrystalized from ethyl acetate to give colored compounds[XV].
2.2.26. Compound[XV]a
- Yield: 45%; m.p. = 213-215℃ (petroleum ether); IR(KBr)
 cm-1: 3421-3160(NH2), 2918-2856(C-H), 1730(C=C), 1610(C=N), 1560, 823 (C-S); Anal. Calcd. for C33H29O9N5S2 (703): C, 56.33; H, 4.13; N, 9.96. Found: C, 56.19; H, 4.01, N, 10.09.
cm-1: 3421-3160(NH2), 2918-2856(C-H), 1730(C=C), 1610(C=N), 1560, 823 (C-S); Anal. Calcd. for C33H29O9N5S2 (703): C, 56.33; H, 4.13; N, 9.96. Found: C, 56.19; H, 4.01, N, 10.09.2.2.27. Compound[XV]b
- Yield: 45%; m.p. = 198-200℃ (petroleum ether); IR(KBr)
 cm-1: 3305-3109(NH2), 2924-2850(C-H), 1693(C=C), 1577(C=N), 817 (C-S); Anal. Calcd. for C39H33O10N5S2 (795): C, 58.87; H, 4.15; N, 8.81. Found: C, 58.77; H, 4.09, N, 8.41.
cm-1: 3305-3109(NH2), 2924-2850(C-H), 1693(C=C), 1577(C=N), 817 (C-S); Anal. Calcd. for C39H33O10N5S2 (795): C, 58.87; H, 4.15; N, 8.81. Found: C, 58.77; H, 4.09, N, 8.41.2.2.28. Preparation of Diphenylimidazoles Derivatives[XVI] a,b
- To a stirred solution of compound[XV] (10 mmol) in dry DMF (10 mL), the benzoin (10 mmol) was added. The reaction mixture was refluxed for 5 hrs. After cooling, drops of water were added with stirring until a precipitate separated out. The precipitate was filtered, dried and recrystallized from petroleum ether.
2.2.29. Compound[XVI]a
- Yield: 50%; m.p. = 90℃ (petroleum ether); IR(KBr)
 cm-1: 3317(NH2), 2924-2852(C-H), 1678(C=C), 1595(C=N), 875 (C-S); Anal. Calcd. for C47H37O9N5S2 (879): C, 64.16; H, 4.21; N, 7.96. Found: C, 64.24; H, 4.19; N, 8.09.
cm-1: 3317(NH2), 2924-2852(C-H), 1678(C=C), 1595(C=N), 875 (C-S); Anal. Calcd. for C47H37O9N5S2 (879): C, 64.16; H, 4.21; N, 7.96. Found: C, 64.24; H, 4.19; N, 8.09.2.2.30. Compound[XVI]b
- Yield: 50%; m.p. =130-132℃ (petroleum ether); IR(KBr)
 cm-1: 3228(NH2), 2926-2848(C-H), 1680(C=C), 1590(C=N), 840 (C-S); Anal. Calcd. for C53H41O11N5S (855): C, 74.39; H, 4.80; N, 8.19. Found: C, 74.53; H, 4.71; N, 8.29.
cm-1: 3228(NH2), 2926-2848(C-H), 1680(C=C), 1590(C=N), 840 (C-S); Anal. Calcd. for C53H41O11N5S (855): C, 74.39; H, 4.80; N, 8.19. Found: C, 74.53; H, 4.71; N, 8.29.3. Results and Discussion
- 5,6-O-isopropylidene-L-ascorbic acid[I] was prepared by the reaction of L-ascorbic acid with acetone in dry HCl[16]. Compound[I] reacts with excess of anisoyl chloride in dry pyridine to give the corresponding ester[II]. Glycols[III] oxidized by periodate, which is cleaves the C5-C6 bond (bearing OH groups) and formation the aldehyde compound D-erythroascorbic acid[IV][22-24]. Methyl-4 - methoxy benzoate[V] was obtained by esterification of 4-methoxy benzoic acid (anisic acid) with methanol[25]. The reaction of 4-methoxybenzoate with hydrazine hydrate in ethanol under reflux give 4-methoxybenzoyl hydrazine[VI] in good yield. Condensation of acid hydrazid with 4-aminobenzoic acid in the presence of dehydrating agent phosphorus oxychloride yielded the oxadiazole derivative [VII]. The reaction of tuloic acid with thiosemicarbazide in the presence of phosphorous oxychloride under reflux for 8 hrs leads to formation 2-amino-5-(4′-tolyl)-1,3,4-thiodiazole [VIII]. The novel Schiff bases[VIII] and[XIII] were synthesized by refluxing equemolare of D-erythroascorbic acid[VI] with amino compounds[VII] or[XII] in dry benzene with some drops of glacial acetic acid. Compounds[IX] or[XIV] were synthesized by refluxing acetyl or anisoyl chloride with Schiff bases[XIII] or[XIII] in dry benzene. The reaction is initiated by attack of the azomethine nitrogen at the carbonyl group of acid chloride (acetyl or anisoyl chloride)[26], displacing the chlorine as a chloride anion and forming the iminium cation[A] which can be represented by structure[B] too. In both structure[A] and[B] the positively charged on nitrogen in[A] and the positively charged on carbon in[B] are linked to strong electron withdrawing groups and are unlikely to form, therefore, N-acyl derivatives are best represented by the covalent structure[C] (Scheme 4).
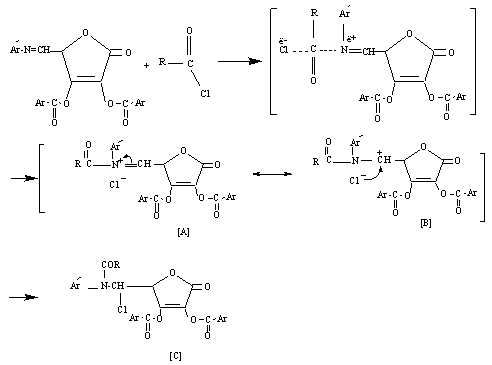 | Scheme 4. Mechanism for the synthesis of compounds[IX] or[XIV] |
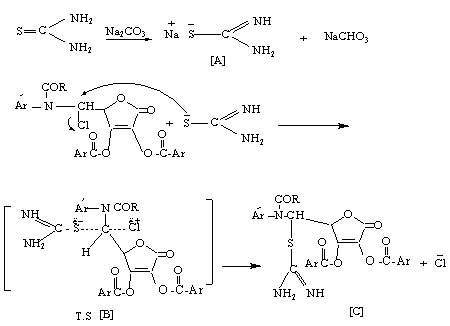 | Scheme 5. Mechanism for the synthesis of compounds[X] or[XV] |
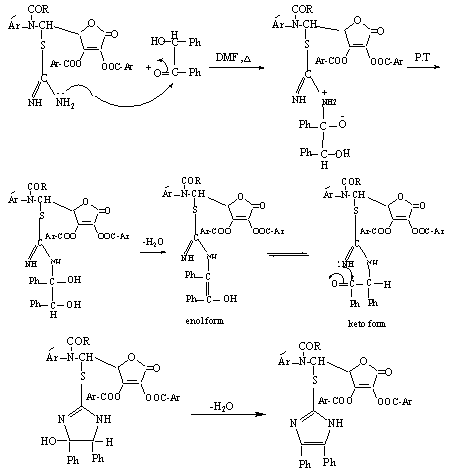 | Scheme 6. Mechanism for the synthesis of compounds[XI] or[XVI] |
3.1. Biological Activity
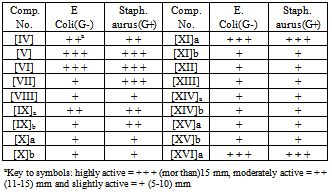
- The antibacterial activity of the synthesized compounds was performed according to the agar diffusion method[27]. The synthesized compounds were tested against E.coli and Staph. aureus. Each compound was dissolved in DMSO to give concentration 1ppm. The plates were then incubated at 37℃ and examined after 24 hrs. The zones of inhibition formed were measured in millimeter and are represented by (-), (+), (+ +) and (+ + +) depending upon the diameter and clarity as in Table 1. All the compounds exhibit the highest or low biological activity against both the organisms. Compounds showed good inhibition against of the two types of the bacteria, this could be related to the presence of the D-erythroascorbic acid, imine linkage or imidazole ring.
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML