-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(6): 171-181
doi:10.5923/j.ajoc.20120206.07
Heterocyclic Synthesis Via Enaminonitriles: One-Pot Synthesis of Some New Pyrazole, Pyrimidine, Pyrazolo[1,5-A]Pyrimidine and Pyrido[2,3-D]Pyrimidine Derivatives
Mohamed A. Khalil, Samia M. Sayed, Mohamed A. Raslan
Chemistry Department, Faculty of Science, Aswan University, 81528 Aswan, Egypt
Correspondence to: Mohamed A. Khalil, Chemistry Department, Faculty of Science, Aswan University, 81528 Aswan, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A simple route for the synthesis of 5-aminopyrazole derivatives (9) was described through the readily accessible acrylamide derivative (7). Reaction of the aminopyrazole with different reagents such as α,β-unsaturated nitriles afforded pyrazol[1,5-a]pyrimidine (13,15,18). The structure of the enaminonitrile (6) was studied with different reagents such as thiourea and 2-thioxo-(1H)-pyrimidine-4-one (36) afforded pyrimidinethione derivative (35) and the pyrido[2,3-d]pyrimidine derivative (41), respectively.
Keywords: DMF-DMA, 5-Aminopyrazole, Enaminonitrile, Thiourea and Pyrimidine
Cite this paper: Mohamed A. Khalil, Samia M. Sayed, Mohamed A. Raslan, Heterocyclic Synthesis Via Enaminonitriles: One-Pot Synthesis of Some New Pyrazole, Pyrimidine, Pyrazolo[1,5-A]Pyrimidine and Pyrido[2,3-D]Pyrimidine Derivatives, American Journal of Organic Chemistry, Vol. 2 No. 6, 2012, pp. 171-181. doi: 10.5923/j.ajoc.20120206.07.
1. Introduction
- Pyrazolo[1,5-a]pyrimidine are of considerable chemical and pharmacological importance as purine analogs and many derivatives of pyrazolo[1,5-a]pyrimidines have been reported to exhibit cyotoxic activity[1-9]. Several 7-substituted pyrazolo[1,5-a]pyrimidine derivatives were reported to have antiproliferative activity against HCT116 and other cell lines (e.g. compounds 1-3)[3,6,8] (figure 1). Also, almost high percentage of adults between ages 40-70 suffer from insomnia at least one time during their lives. The drug Zaleplon (4)[10-13] has been found to be efficient in the treatment of sleep disorder where difficulty in falling asleep is the primary issue. Unlike many other hypnotic drugs, this substance does not interfere with sleep architecture and can be administered for up to five weeks without the risk of dependence or rebound insomnia upondiscontinuation. Indiplon (5)[10, 14] has recently been released for use for the same purpose while the developments of Ocinaplon (6)[15], which is an anxiolytic drug in the pyrazolopyrimidine family of drugs, has been discontinued owing to liver complications observed in clinical trials. As a result, a need exists for the development of analogs of (1-6). (figure 1).The present work comprises the combination of benzimidazole pharmacophore with various substituted aromatic or heterocyclic rings via carboxamides linker. It was interesting to synthesis hybrids possessing biological and pharmacological activities due to benzimidazole[16-29] and certain substituted pyrazoles[30-44] hoping that this combination may possess a potential analgesic activity along with possible anticancer effect.In connection with our previous studies[45-53] and in view of utilizing the cyan-oacetamide as highly versatile intermediates for the construction of functionlized pyrazole, pyrimidinethione pyrazolopyrimidine and pyridopyrimidine derivatives of expected potential biological activity and excellent pharmacology encouraged us to synthesis novel entitled derivatives.
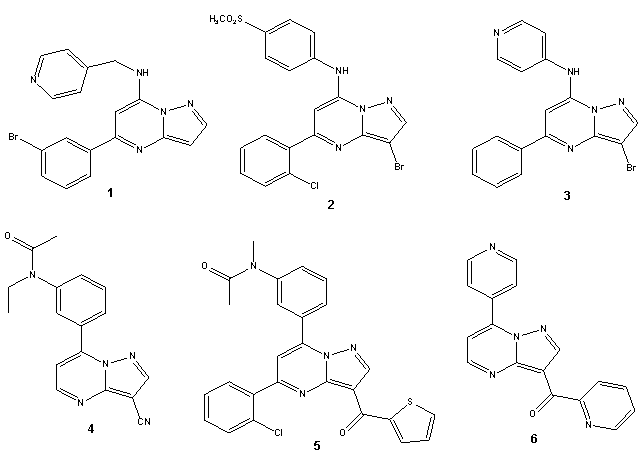 | Figure 1. 7-Substituted pyrazolo[1,5-a]pyrimidine suc has Zaleplon 4, Indiplon 5 and Ocinaplon 6 |
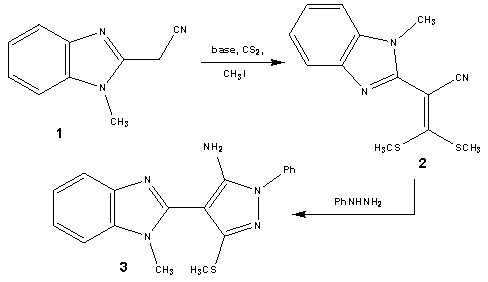 | Scheme 1. Synthesis of 5-aminopyrazole derivative (3) |
2. Chemistry
- Treatment of 2-(1-methyl-1H-benzo[d]imidazol-2-yl)-3, 3-bis-(methylthio)acrylonitrile (2) with phenylhydrazine affords the target4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3- (methylthio)-1-phenyl-1H-pyrazol-5-amine (3). The structure of (3) was esta-blished and confirmed as the reaction product on the basis of their elemental analysis and spectral data. The IR spectrum showed absorption band in the region 3285 cm-1 assignable for NH2, in addition to disappearance of nitrile function signal. Its 1H NMR spectrum revealed the presence of singlet signals at δ 2.62 ppm, δ 3.98 ppm and δ 5.72 ppm assignable to the SCH3, N-CH3 and NH2 protons, respectively. Its mass spectrum showed a molecular ion peak at m/z = 335 corresponding to a molecular formula C18H17N5S. ( Scheme 1).Treatment of4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-amine (3) with (3,5-dimethy-1H- pyrazol-1-yl)-3-oxopropanenitrile as cyanoacetylation reagent in dry toluene afforded 2-cyano-N-(4-(1- methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acetamide (4). The structure of (4) was established on the basis of spectral data. The IR spectrum revealed absorption band at 3215 cm-1 for the NH group, sharp band at 2218 cm-1 for the cyano function and strong band at 1658 cm-1 for carbonyl group. Its 1H NMR spectrum revealed the presence of singlet signals at δ 4.12 ppm and δ 10.65 ppm assignable to the methylene and NH protons, respectively. Its mass spectrum showed a molecular ion peak at m/z = 402 corresponding to a molecular formula C21H18N6OS. The reactivity of 2-cyano-N-(4-(1-methyl- 1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl) acetamide (4) to give pyrazole, pyrimidinethione pyrazolopyrimidine and pyridopyrimidine derivatives was investigated.Treatment of2-cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl) acetamide (4) with DMF-DMA (5) in refluxing toluene afforded the corresponding2-cyano-3-(dimethylamino)-N-(4-(1-methyl-1H-benzo[d]-imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acrylamide (6). The struct-ure of the enaminonitrile (6) has been assigned as a reaction product on the basis of analytical and spectral data. The IR spectrum displayed absorption bands at 3240 cm-1 due to the NH function, 2198 cm-1 due to the conjugated cyano function, 1663 cm-1 due to the amidic carbonyl function. The 1H NMR spectrum exhibited two sharp singlet signals at δ 3.21 ppm and δ 3.35 ppm assignable to N,N-dimethylamino protons, anther singlet signal at δ 8.09 ppm specific for methane proton and broad signal at δ 11.47 ppm due to NH proton. The mass spectrum showed a molecular ion peak at m/z = 457, corresponding to molecular formula C24H23N7OS. (scheme 2).The behavior of the enaminonitrile (6) toward some N-nucleophiles to attain polyfunctionally substituted azoles, azines and related fused systems linked to pyrazole moiety through a carboxamide linkage of potential pharmaceutical interest, has been investigated. Treatment of (6) with hydrazine hydrate at room temperature afforded 2-cyano-3-hydrazinyl-N- (4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acrylamide (7). The structure of acyclic hydrazine derivative (7) has been assigned as a reaction product on the basis of analytical and spectral data. The IR spectrum displayed absorption bands at 3410 cm-1, 3280 cm-1, 3218 cm-1, 3180 cm-1 due to the NH2 and NH functions, 2193 cm-1 due to cyano function, 1671 cm-1 due to the amidic carbonyl function. The 1H NMR spectrum exhibited four singlet signals at δ 5.04 ppm, δ7.78 ppm, δ 8.85 ppm and δ 10.25 ppm assignable to NH2, olefinic CH, 2NH protons, respectively. The mass spectrum showed a molecular ion peak at m/z = 444, corresponding to molecular formula C22H20N8OS. (scheme 3).Refluxing of the acrylamide derivative (7) with stirring in ethanol gave the cyanopyrazole (8). The structure of the later has been assigned as a reaction product on the basis of analytical and spectral data. The IR spectrum displayed absorption bands at 3270 cm-1, 3164 cm-1 due to the (2NH) functions, 2228 cm-1 due to cyano function. The 1H NMR spectrum of (8) exhibited three singlet signals at δ 8.34 ppm, δ 13.04 ppm and δ 9.61 ppm assignable to 2NH and pyrazole-CH protons, respectively. The mass spectrum showed a molecular ion peak at m/z = 426, corresponding to molecular formula C22H18N8S. (scheme 3).When the acrylamide (7) refluxed in pyridine solution, cyclization gradually took place via addition of amino of hydrazine to the cyano function afforded 5-aminopyrazole derivative (9) which achieved as a sole product. The structure of (9) has been assigned as a reaction product on the basis of analytical and spectral data. The IR spectrum displayed absorption bands at 3430 cm-1, 3354 cm-1 due to NH2 function, 3275 cm-1, 3129 cm-1 due to the NH function, 1659 cm-1 due to the amidic carbonyl function. Inspection of 1H NMR spectrum enabled establishing structure (9) for this pyrazole derivative since the pyrazole H-3 appeared as a singlet signal at δ 8.19 ppm. We could not trace in the 1H NMR spectrum any signals for the tautomeric 3-amino-pyrazole (10) as this could reveal pyrazole-H5 as a doublet. The mass spectrum showed a molecular ion peak at m/z = 444, corresponding to molecular formula C22H20N8OS. (scheme 3).The structure of 5-aminopyrazole derivative (9) was further confirmed by its alternate synthesis via stirring at 50-60℃ of 2-cyano-3-(dimethylamino)-N- (4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acryl-amide (6) with hydrazine hydrate which afforded product identical in all respects (mp, mixed mp, TLC and spectral data IR, 1H NMR and MS) with those of 5-aminopyrazole derivative (9) (scheme 3).
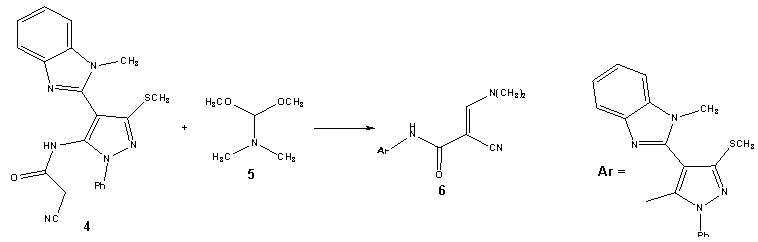 | Scheme 2. Synthesis of acrylamide derivative (6) |
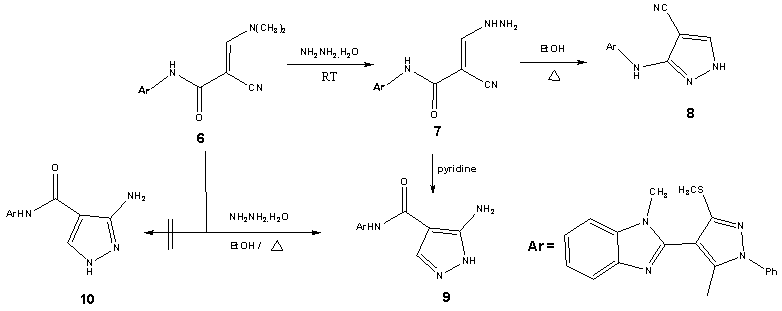 | Scheme 3. synthesis of pyrazole derivatives (8) and (9) |
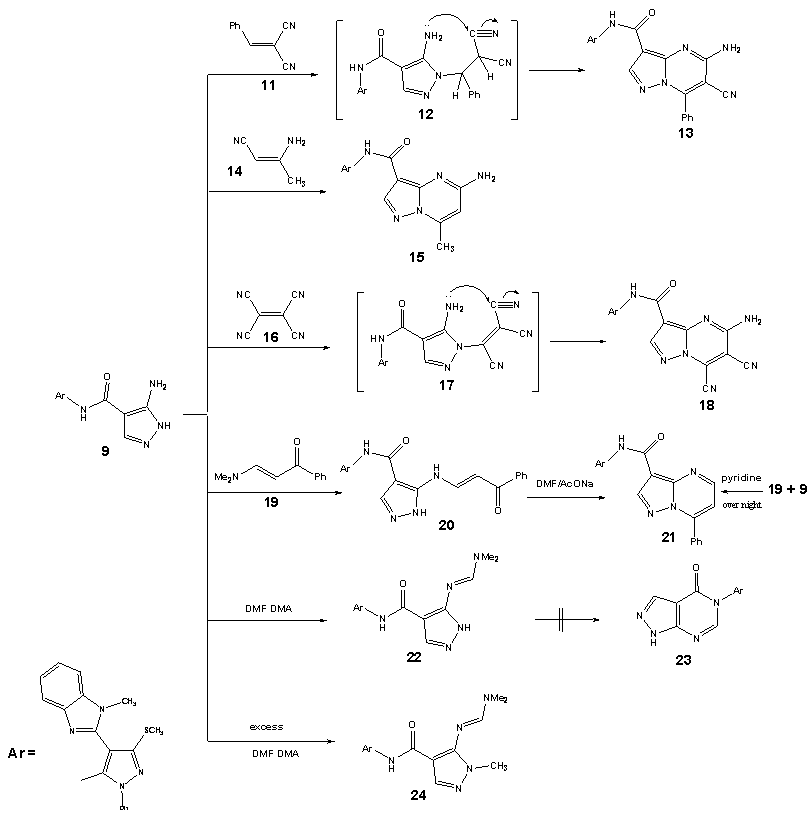 | Scheme 4. synthesis of pyrazolopyrimidine derivatives 13, 15, 18 and 21 |
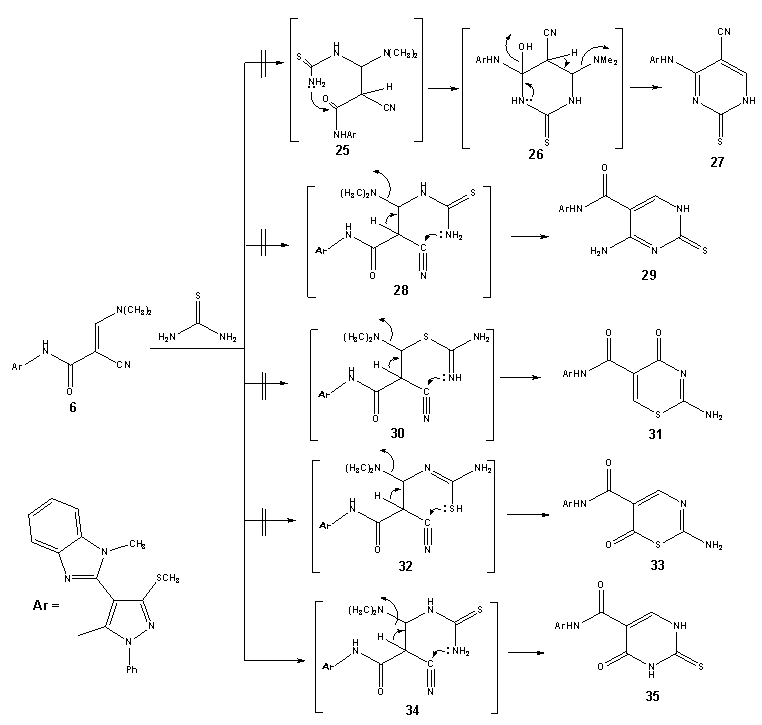 | Scheme 5. synthesis of thioxopyrimidine (35) |
3. Experimental
- All organic solvents were purchased from commercial sources and used as received or dried using standard procedures, unless otherwise stated. All chemical were purchased from Aldrich or Across and used without purification. Melting points were measured on a Gallenkamp apparatus and are uncorrected. IR spectra were recorded on Shimadzu FT-IR 8101 PC infrared spectrophotometer. The 1H NMR spectra were determined in DMSO-d6 at 300 MHz on a Varian Mercury VX 300 NMR spectrometer using TMS as an internal standard. Mass spectra were measured on a GCMS-QP1000 EX spectrometer at 70Ev. Elemental analyses were carried out at the Microanalytical Center of Cairo University. (3, 5-Dimethy-1H-pyrazol-1-yl)-3- oxopropanenitrile was prepared according to the reported literature[58]. 4-(1-Methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-amine (3) was prepared according to the reported literature [59].2).2-Cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acetamide (4).A solution of 4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3- (methylthio)-1-phenyl-1H-pyrazol-5-amine (3) (0.01 mol) in dry toluene (30 ml) was added to solution of with (3,5-dimethy-1H-pyrazol-1-yl)-3-oxopropanenitrile (0.01 mol) in dry toluene (30 ml). The reaction mixture was refluxed for about 1h. After evaporation of the solvent, the solid product was collected by filtration and recrystallised from dry DMF to afford (83%, yield) of (4), mp 293-295°C; IR υmax / cm-1 (KBr) 3215, 3172 (NH), 2218 (CN), 1658 (CO), 1630 (C=N); 1H NMR (DMSO-d6) δ 2.68 (s, 3H, SCH3), 3.91 (s, 3H, NCH3), 4.12 (s, 2H, CH2), 7.51-8.01 (m, 9H, Ar-H), 10.65 (s, 1H, NH); m/z 402 (M+, 13.75). Anal. Calcd. For C21H18N6OS (402.47): C, 62.67; H, 4.51; N, 20.88; S, 7.97%. Found: C, 62.63; H, 4.49; N, 20.78; S, 7.94%.3).2-Cyano-3-(dimethylamino)-N-(4-(1-methyl-1H-benzo[d]-imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acrylamide (6).A mixture of 2-cyano-N-(4-(1-methyl-1H-benzo[d] imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acetamide (4) (10 mmol) and DMF/DMA (5) (1.2g, 10mmol) in. dry toluene.(25 ml) was stirred at room temperature for 6h. The separated solid product formed on standing at room temperature. The pale orange precipitate product was filtered off, washed with diethyl ether, dried and recrystallized from toluene to give (84%, yield) of (6), mp 256°C; IR υmax / cm-1 (KBr) 3240 (NH), 2198 (CN), 1663 (amidic C=O), 1630 (C=N); 1H NMR (DMSO-d6) δ 2.67 (s, 3H, SCH3), 3.21 (s, 3H, NCH3), 3.35 (s, 3H, NCH3), 3.94 (s, 3H, NCH3), 7.25-7.93 (m, 9H, Ar-H), 8.09 (s, 1H, CH=), 11.47 (s, br, 1H, NH); m/z 457 (M+, 29.12). Anal. Calcd. For C24H23N7OS (457.55): C, 63.00; H, 5.07; N, 21.43; S, 7.01%. Found: C, 62.96; H, 5.02; N, 21.40; S, 7.10%.4).2-Cyano-3-hydrazinyl-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acrylamide (7).A solution of the enaminonitrile (6) (10mmol) and hydrazine hydrate (80%. 0.65 ml) in ethanol (40 ml) were stirred at reflux for 30 minutes, then cooled to room temperature. The solid product was separated by filtration, washed with ethanol, and recrystallized from dioxan as white crystals to give (69%, yield) of (7), mp 231°C; IR υmax / cm-1 (KBr) 3410, 3280, 3218, 3180 (NH2 and 2NH), 2193 (CN), 1671 (amidic C=O), 1628 (C=N); 1H NMR (DMSO-d6) δ 2.69 (s, 3H, SCH3), 3.92 (s, 3H, NCH3), 5.04 (s, br, 2H, NH2), 7.78 (s, 1H, CH=), 7.21-7.97 (m, 9H, Ar-H), 8.85 (s, br, 1H, NH), 10.25 (s, br, 1H, NH); m/z 445 (M++1, 28.6), 444 (M+, 100). Anal. Calcd. For C22H20N8OS (444.51): C, 59.44; H, 4.54; N, 25.21; S, 7.21%. Found: C, 59.42; H, 4.56; N, 25.18; S, 7.17%.5).3-(4,5-Dihydro-4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-ylamino)-1H-pyrazole-4-carbonitrile (8).A mixture of the enaminonitrile (7) (10 mmol) and hydrazine hydrate (80%. 0.65 ml) in ethanol (40 ml) stirred at reflux for 4h., then concentrated in vacuo to one third of its volume, cooled to room temperature, poured onto ice/water. The solid product which formed was collected by filtration, washed with water several times and recrystallized from dioxan to give (76%, yield) of (8), mp 261-263°C; IR υmax / cm-1 (KBr) 3270, 3164 (2NH), 2228 (CN), 1632 (C=N); 1H NMR (DMSO-d6) δ 2.70 (s, 3H, SCH3), 3.95 (s, 3H, NCH3), 7.31-8.02 (m, 8H, Ar-H), 8.34 (s, br, 1H, NH), 9.61 (s, 1H, pyrazole H-5), 13.04 (s, br, 1H, NH), 10.25 (s, br, 1H, NH); m/z 427 (M++1, 12.7), 426 (M+, 37). Anal. Calcd. For C22H18N8S (426.5): C, 61.95; H, 4.25; N, 26.27; S, 7.52%. Found: C, 61.86; H, 4.23; N, 26.26; S, 7.54%.
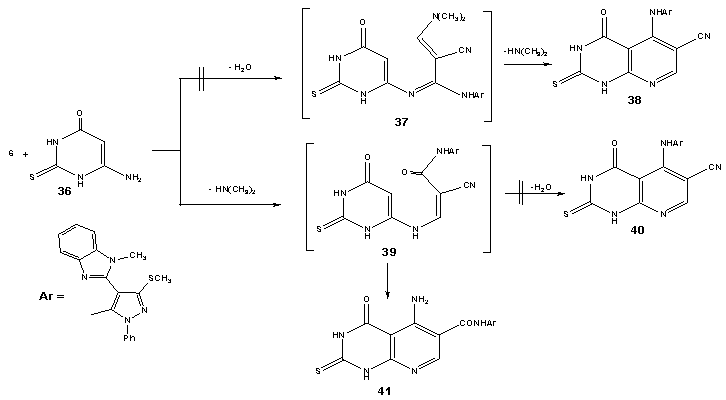 | Scheme 6. synthesis of 2-thioxo pyrido[2,3-d] pyrimidin-4-one derivative (41) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML