-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(6): 161-170
doi: 10.5923/j.ajoc.20120206.06
Reactivity of 2-Cyano-N-(4-(1-Methyl-1H-benzo[d]imidazol-2-yl)-3-(Methylthio)-1-Phenyl-1H-Pyrazol-5-yl)Acetamide: A Facile Synthesis of Pyrazole, Thiazole, 1,3,4-Thiadiazole and Polysubstituted Thiophene Derivatives
Mohamed A. Khalil , Samia M. Sayed , Mohamed A. Raslan
Chemistry Department, Faculty of Science, Aswan University, 81528 Aswan, Egypt
Correspondence to: Mohamed A. Raslan , Chemistry Department, Faculty of Science, Aswan University, 81528 Aswan, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Treatment of 2-cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl) acetamide (4) with phenyl isothiocyanate gave the thiole derivative (6) which on treatment with hydrazonyl chlorides (7a-c) furnished 1,3,4-thiadiazole derivatives (9a-c). Reaction of cyanoacetamide derivative (4) with active methylene reagents such as malononitrile or ethyl cyanoacetate and elemental sulfur afforded the corresponding polysubstituted thiophene derivatives (18a,b). Reaction of cyanoacetamide derivative (4) with benzaldehyde yielded the phenylmethylidene derivative (21). The latter showed interesting reactivity towards cyanomethylene reagent and hydrazine derivatives afforded pyrane (22a,b) and pyrazole (25a,b) derivatives.
Keywords: Cyanoacetamide, Active Methylene, Hydrazonyl Halides , α-halo-carbonyl Compounds
Cite this paper: Mohamed A. Khalil , Samia M. Sayed , Mohamed A. Raslan , "Reactivity of 2-Cyano-N-(4-(1-Methyl-1H-benzo[d]imidazol-2-yl)-3-(Methylthio)-1-Phenyl-1H-Pyrazol-5-yl)Acetamide: A Facile Synthesis of Pyrazole, Thiazole, 1,3,4-Thiadiazole and Polysubstituted Thiophene Derivatives", American Journal of Organic Chemistry, Vol. 2 No. 6, 2012, pp. 161-170. doi: 10.5923/j.ajoc.20120206.06.
Article Outline
1. Introduction
- Several pyrazole derivatives are important pharmaceutical, they have been found to possess diverse biological activities [1-6]. They are also acknowledged for their anticancer[7-11], antipyretic[12], anti-inflammatory[13], antimicrobialactivities[14-16], antiviral[17], tranquillizing [18],antihypertensive[19], antidepressant[20], antiarrhythmi [21], psychoanalep tic[22], anticonvulsant[23] and antidiabetic activities[24].Moreover, the thiazole derivative has received a great deal of attention due to their pharmacological importance. Thiazoles were reported to possess antimicrobial[25-28], analgesic[29], anti-inflammatory[30], anticonvulsant[31], cardiotonic[32], anticancer[33,34], antitubercular[35] and anthelmintic[36] activites. Antimicrbial activities of some substituted thiazoles are well established because it posses (S-C=N) toxophoric unit. Thiazoles have enhanced lipid solubility with hydrophilicity. Also, thiazoles are easily metabolized by routine biochemical reactions and are non-carcinogenic in nature[37]. many thiophene containing annulated compoun ds, exhibite biological activities[38,39].In addition, benzimidazole has been an important pharmacophore and privileged structure in medicinal chemistry[40] encompassing a diverse rang of biological activities including antiarrhythmic, antiulcer, antihistamine, antifungal, antiviral and cytotoxicity[41]. Furthermore, 1,3,4 -thiadiazoles were reported as highly anti- inflammatory[42], antimicrobial[43], anticonvulsant[44] and anticancer[45]. agents.Cyanoacetamides are highly reactive compounds. They are extensively utilized as reactants or reaction intermediate since the carbonyl and the cyano functions of these compounds are suitably situated to enable reactions with common bidentate reagents to form a variety of heterocyclic compounds. Moreover function, the active hydrogen on C-2 of these compounds can take part in a variety of condensation and substitution reactions. The synthesis of cyanoacetamides may be carried out in several ways[46, 47]. Cyanoacetyl pyrazole is very handly and cheap cyanoacetylation reagent It was successfully applied for the synthesis of various N-alky and N-aryl cyanoecatamides. The latter are polyfunctional compounds possessing both electrophlic and nucleophlilic properties. Cyanoacetamide can be useful for the synthesis of three-six membered rings[48].In connection with our previous studies[49-58] and in view of utilizing the cyanoacetamide as highly versatile intermediates for the construction of functionlized pyrazole, thiazole, 1,3,4-thiadiazole and polysubstituted thiophene derivatives of expected potential biological activity and excellent pharmacology encouraged us to synthesis novel entitled derivatives.
2. Chemistry
- Treatment of 2-(1-methyl-1-H-benzo[d]imidazol-2-yl)-3, 3-bis-(methylthio)acrylonitrile (2) with phenylhydrazine affords the target4-(1-methyl-1H-benzo[d]imidazol-2- yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-amine (3). The structure of (3) was established and confirmed as the reaction product on the basis of their elemental analysis and spectral data. The IR spectrum showed absorption band in the region 3285 cm-1 assignable for NH2, in addition to disappearance of cyano function signal. Its 1H NMR spectrum revealed the presence of singlet signals at δ 2.62 ppm, δ 3.98 ppm and δ 5.72 ppm assignable to the SCH3, N-CH3 and NH2 protons, respectively. Its mass spectrum showed a molecular ion peak at m/z = 335 corresponding to a molecular formula C18H17N5S. ( Scheme 1)Treatment of 4-(1-methyl-1H-benzo[d]imidazol-2-yl)- 3-(methylthio)-1-phenyl-1H-pyrazol-5-amine (3) with (3,5- dimethy-1H- pyrazol -1-yl)-3-oxopropanenitrile as cyanoac etylation reagent in dry toluene afforded 2-cyano-N-(4- (1-methy l-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phe nyl-1H-pyrazol-5-yl)acetamide (4). The structure of (4) was established on the basis of spectral data. The IR spectrum revealed absorption band at 3215 cm-1 for the NH group, sharp band at 2218 cm-1 for the cyano function and strong band at 1658 cm-1 for carbonyl group. Its 1H NMR spectrum revealed the presence of singlet signals at δ 4.12 ppm and δ 10.65 ppm assignable to the methylene and NH portons, respectively. Its mass spectrum showed a molecular ion peak at m/z = 402 corresponding to a molecular formula C21H18N6OS. The reactivity of2-cyano-N-(4-(1-methyl- 1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyr-azol-5-yl) acetamide (4) to give pyrazole, thiazole, thiophene and 1,3,4-thiadiazole derivatives was investigated. Thus, reaction of (4) with phenyl isothiocyanate in DMF and potassium hydroxide solution afforded the corresponding non-isolable enaminonitrile (5) which converted (6A) or (6B) form upon treatment with dilute hydrochloric acid (scheme 2). 1H NMR spectrum of the reaction product (6) was free from any aliphatic protons which mean that compound (6) is solely present in (6B) form instead of its tautomer (6A). The IR spectrum of (6) revealed absorption bands at 3362 cm-1, 3215 cm-1 for the NH groups, absorption at 2206 cm-1 for the cyano function and absorption band at 1653 cm-1 for carbonyl group. Its 1H NMR spectrum revealed the presence of two singlet signal at δ 9.85 ppm, δ 10.65 ppm assignable to the NH protons and singlet signal at δ 13.84 ppm assignable to the SH group. Its mass spectrum showed a molecular ion peak at m/z = 537 corresponding to a molecular formula (C28H23N7OS2).Compound (6) is a versatile multifunctional reagent and its reactivity towards hydrazonyl chloride (7a-c) was studied. Thus, reaction of compound (6) withC-phenyl-N- phenylhydrazonyl chloride (7a) in refluxing ethanol solution containing triethylamine as basic catalyst afforded solely the corresponding 1,3,4-thiadiazole derivatives (9a-c). Formation of the latter structures is assumed proceed via elimination of aniline molecule from the non-isolable intermediate (8) as outlined in scheme 2. The 1, 3, 4-thiadiazole structure derivative (9a-c) was confirmed from the elemental analyses and spectral data of the isolated product. The IR spectrum of (9a) revealed absorption band at 2208 cm-1 assignable to cyano function and absorption band at 1662 cm-1 assignable to carbonyl group. Its 1H NMR spectrum spectrum revealed singlet signal for NH at δ 9.98 ppm in addition to multiplet signal for aromatic protons at δ 7.37-8.12 ppm. Its mass spectrum showed a molecular ion peak at m/z = 638 corresponding to a molecular formula (C35H26N8OS2).In similar manner compound (6) reacted with equimolar amounts of C-acetyl-N-phenylhydrazonyl chloride (7b) and C-ethyl carboxylate-N-phenylhydrazonyl chloride (7c) frunished in each case one isolable product (as tested by TLC analyses) which have the 1,3,4-thiadiazole structures (9b,c), respectively based on their elemental and spectral analyses (scheme 2).On the other hand we investigate the reactivity of non-isolable enaminonitrile (5) with α-halocarbonyl compounds (10a-c). These reactions may be lead to formation of either thiazole or thiophene systems or both depending on the reaction conditions and the nature of the α-halocarbonyl reagent[59]. Thus, reaction of (5) in situ with α-halo-carbonyl compounds such as bromoacetone, ethyl bromoacetate and 1-(benzothiazol-2-yl)-2-bromoethanone (10a-c), respectively resulted in the formation of single product for which the thiazole (12), (13b,c) or thiophene (14) structures, can be assumed. However the elemental and spectral data of the reaction product were incomplete accordance with the thiazole derivatives (12) and (13b,c). The IR spectrum for (12) displayed stretching absorption band at 3347 cm-1and 2223 cm-1 for the NH and cyano function, respectively and two carbonyl absorption band at 1685 and 1648 cm-1. Its 1H NMR spectrum displayed singlet signals at δ 10.31 ppm assignable to the NH porton and singlet signal at δ 3.73 ppm assignable to the methylene protons. The IR spectrum of (13b) displayed stretching absorption band at 3218 cm-1 and 2204 cm-1 for the NH and cyano function. Its 1H NMR spectrum revealed new signal at δ 1.72 ppm for methyl portons and δ 6.93 ppm assignable for thiazole =CH proton and singlet signal at δ 10.53 ppm assignable to the NH group. Its mass spectrum showed a molecular ion peak at m/z = 575 corresponding to a molecular formula (C31H25N7OS2).The mass spectrum of compounds (12) and (13b,c) showed a molecular ion peak at m/z = 577, 575 and 694 corresponding to the molecular formula (C30H23N7O2S2), (C31H25N7OS2), and (C37H26N8OS3), respectively. (scheme 3)2-cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-Pyrazol-5-yl)acetamide (4) unde rwent Gewald thiophene synthesis via its reaction with cyanomethylene derivatives (15a,b) and elemental sulfur in refluxing dioxane containing triethylamine as basic catalyst afforded the 3,5-diamino thiophene derivatives (18a,b), respectively. Formation of (18a,b) proceed via non-isolable intermediates (16) and (17). The analytical and spectral data of compounds (18a,b) are consistent with the proposed structures. The IR spectrum of (18a) displayed absorption bands at 3316, 3218 cm-1 and 2198 cm-1 for the NH2 and cyano function and carbonyl absorption band at 1662 cm-1. Its 1H NMR spectrum two singlet signals at δ 4.42 ppm and δ 5.56 ppm for NH2 protons and singlet signal at δ 10.21 ppm assignable to the NH group. Its mass spectrum showed a molecular ion peak at m/z = 500 corresponding to a molecular formula (C24H20N8OS2).On the other hand reaction of (4) with phenyl isothiocayanate and elemental sulfur afforded the 4-amino-2,3-dihydro-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(me-thylthio)-1-phenyl-1H-pyrazol-5-yl)-3-phenyl-2-thioxothiazole-5-carboxamide (20) which proceed via intermediacy (16) and (19). The analytical and spectral data of compounds (18a,b) and (20) are consistent with the proposed structures (scheme 4).
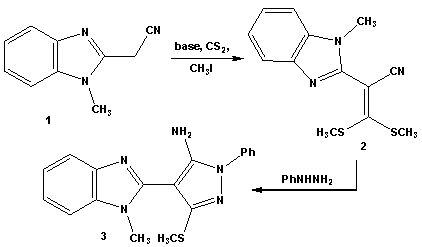 | Scheme 1. Synthesis of 5-aminopyrazole derivative 3 |
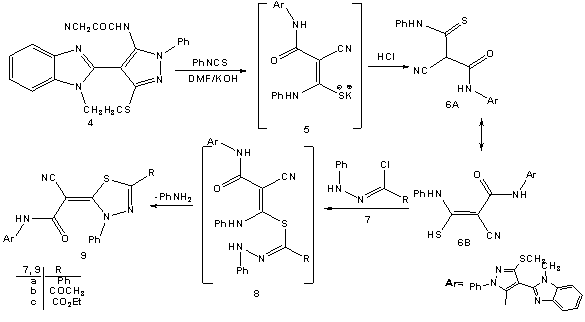 | Scheme 2. Synthesis of 1,3,4-thiadiazole dericatives 9a-c |
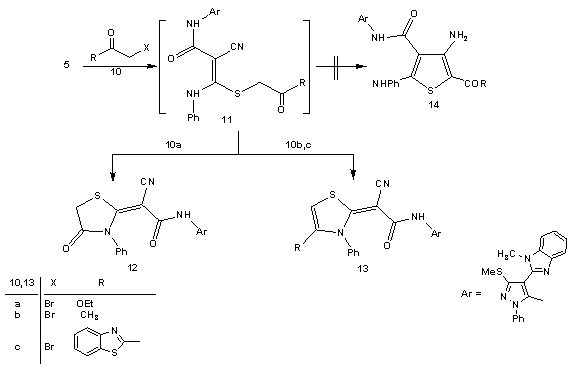 | Scheme 3. Reactions of halocarbonyl compounds 10a-c with enaminonitriles 11a-c |
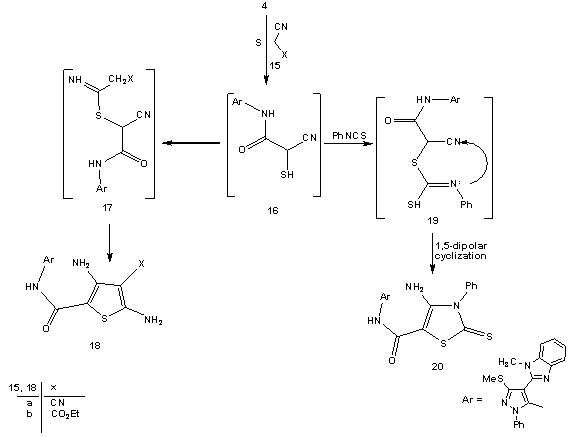 | Scheme 4. Synthesis of polysubstituted thiophene 18a,b and thiazole 20 derivatives |
 | Scheme 5. Synthesis of pyrane 22a,b pyrazole derivatives 25a,band 26 |
3. Experimental
- All organic solvents were purchased from commercial sources and used as received or dried using standard procedures, unless otherwise stated. All chemical were purchased from Aldrich or Across and used without purification. Melting points were measured on a Gallenkamp apparatus and are uncorrected. IR spectra were recorded on Shimadzu FT-IR 8101 PC infrared spectrophotometer. The 1H NMR and 13C NMR spectra were determined in DMSO-d6 at 300 MHz on a Varian Mercury VX 300 NMR spectrometer using TMS as an internal standard. Mass spectra were measured on a GCMS-QP1000 EX spectrometer at 70Ev. Elemental analyses were carried out at the Microanalytical Center of Cairo University. (3,5-Dimethy-1H-pyrazol-1-yl)-3-oxopropanenitrile was prepared according to the reported literature[60], Hydrazonyl halides (7a) and (7b,c) were prepared according to the reported literature[61] and[62,63], respectively. 4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-Phenyl-1H-pyrazol-5-amine (3) was prepared according to the reported literature[64].2).2-Cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acetamide (4).A solution of4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3- (methylthio)-1-phenyl-1H-pyrazol-5-amine (0.01 mol) in dry toluene (30 ml) was added to solution of with (3,5-dimethy -1H-pyrazol-1-yl)-3-oxopropanenitrile (0.01 mol) in dry toluene (30 ml). The reaction mixture was refluxed for about 1h. After evaporation of the solvent, the solid product was collected by filtration and recrystallised from dry DMF to afford (83%, yield) of (4), mp 293-295°C; IR υmax / cm-1 (KBr) 3215, 3172 (NH), 2218 (CN), 1658 (CO), 1630 (C=N); 1H NMR (DMSO-d6) δ 2.68 (s, 3H, SCH3), 3.91(s, 3H, NCH3), 4.12 (s, 2H, CH2), 7.51-8.01 (m, 9H, Ar-H), 10.65 (s, 1H, NH); m/z 402 (M+, 13.75). Anal. Calcd. For C21H18N6OS (402.47): C, 62.67; H, 4.51; N, 20.88; S, 7.97%. Found:. C, 62.63; H, 4.49; N, 20.78; S, 7.94%.3).2-Cyano-3-mercapto-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-methylthio-1-phenyl-1H-pyrazol-5-yl)-3-phenylamino acrylamide (6).To stirred solution of potassium hydroxide (0.01 mol) in dimethylformamide (20ml),2-cyano-N-(4-(1-methyl-1H- benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acetamide (4) (0.01 mol) was added. After stirring for 30 min., phenyl isothiocyanate (0.01 mol) was added to the resulting mixture. Stirring was continued overnight then poured onto crushed ice containing hydrochloric acid. The solid product so-formed was collected by filtration, washed with water, dried and finally recrystallized from ethanol/dimethylformamide to afford (72%, yield) of (6), mp 239-241°C; IR υmax / cm-1 (KBr) 3362, 3215 (NH), 2206 (CN), 1653 (CO), 1635 (C=N); 1H NMR (DMSO-d6) δ 2.68 (s, 3H, SCH3), 3.89 (s, 3H, NCH3), 7.35-7.98 (m, 14H, Ar-H), 9.85 (s, 1H, NH), 10.65 (s, 1H, NH), 13.84 (s, 1H, SH); m/z 538 (M++1, 26.12). Anal. Calcd. For C28H23N7OS2 (537.66): C, 62.55; H, 4.31; N, 18.24; S, 11.93%. Found:. C, 62.53; H, 4.28; N, 18.19; S, 11.99%.
3.1. General Procedure for the Reaction of 2-Cyano-3-mercapto-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-methylthio-1-phenyl-1H-pyrazol-5-yl)-3-phenylamino acrylamide (6) with Hydrazonoyl Chlorides (7a-c)
- To a solution of (6) (1 mmol) in ethanol (20 ml) and the appropriate hydrazonoyl chlorides (7a-c) (1 mmol of each), triethylamine (0.5 ml) was added. The reaction mixture was refluxed for 4h., then allowed to cool. The formed solid was collected by filtration, washed with ethanol and recrystallized from the suitable solvent to afford the corresponding 1, 3, 4-thiadiazole derivatives (9a-c).1).2-Cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-2-(3,5-diphenyl-1,3,4-thiadiazol-2(3H)ylidene)acetamide (9a).This compound was obtained as orange-red powder (DMF/EtOH 1:2), (53%, yield), mp 208-210°C; IR υmax / cm-1 (KBr) 3237 (NH), 2208 (CN), 1662 (CO), 1632 (C=N); 1H NMR (DMSO-d6) δ 2.66 (s, 3H, SCH3), 3.86 (s, 3H, NCH3), 7.37-8.12 (m, 19H, Ar-H), 9.98 (s, 1H, NH); m/z 638 (M+, 28.34). Anal. Calcd. For C35H26N8OS2 (638.76): C, 65.81; H, 4.10; N, 17.54; S, 10.04%. Found:. C, 65.78; H, 4.10; N, 17.52; S, 9.99%.2).2-(5-Acetyl-3-phenyl-1,3,4-thiadiazol-2(3H)-ylidene)-2-cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acetamide (9b).This compound was obtained as brownish-red crystals (DMF/EtOH 1:20), (55%, yield), mp 281-283°C; IR υmax / cm-1 (KBr) 3232 (NH), 2198 (CN), 1650; 1683 (CO), 1630 (C=N); 1H NMR (DMSO-d6) δ 2.69 (s, 3H, SCH3), 2.72 (s, 3H, COCH3), 3.90 (s, 3H, NCH3), 7.34-8.07 (m, 14H, Ar-H), 10.15 (s, 1H, NH); m/z 604 (M+, 21.45). Anal. Calcd. For C31H24N8O2S2 (604.70): C, 61.57; H, 4.00; N, 18.53; S, 10.61%. Found:. C, 61.55; H, 4.10; N, 18.51; S, 10.58%.3).Ethyl 5-((4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3 -(methylthio)-1-phenyl-1H-pyr-azol-5-ylcarbamoyl)(cyano)methylene)-4,5-dihydro-4-phenyl-1,3,4-thiadiazol-2-carboxylate (9c).This compound was obtained as pal brown crystalsr (DMF/EtOH), (42%, yield), mp 241-243°C; IR υmax / cm-1 (KBr) 3234 (NH), 2203 (CN), 1732 (CO), 1655 (CO), 1631 (C=N); 1H NMR (DMSO-d6) δ 1.45 (t, 3H, J = 7.2 Hz, CH3 ester), 2.69 (s, 3H, SCH3), 3.92 (s, 3H, NCH3), 4.52( q, 2H, J = 7.2 Hz, CH2 ester), 7.31-8.11 (m, 14H, Ar-H), 10.18 (s, 1H, NH); m/z 635 (M+, 28.34). Anal. Calcd. For C32H26N8O3S2 (634.73): C, 60.55; H, 4.13; N, 17.65; S, 10.10%. Found:. C, 60.53; H, 4.11; N, 17.61; S, 10.10%.
3.2. General Procedure for the synthesis of 2-Cyano -N-(4-(1-methyl-1H-benzo[d]-imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-2-(4-oxo-3-phenylthiazol-idin-2-ylidene)acetamide (12), 2-Cyano-N-(4 -(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-2-(4-methyl-3-phenylthiazol-2(3H)-ylidene)-acetamide (13b) and 2-(4-(benzo[d]thiazol-2-yl)-3-phenylthiazol-2(3H)-ylidene-2-cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl) acetamide (13c)
- To stirred solution of potassium hydroxide (10 mmol) in dimethylformamide (20ml), 2-cyano-N-(4-(1-methy l-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acetamide (4) (10 mmol) was added. After stirring for 30 min., phenyl isothiocyanate (10 mmol) was added to the resulting mixture. Stirring was continued 6h., and then the appropriate α-halocarbonyl compounds (10a-c) (10 mmol of each) was added. Stirring continued for additional overnight. Then, the reaction mixture was poured onto crushed ice water. The solid product that formed was filtered off, dried and recrystallized from the suitable solvent to afford the corresponding thiazole derivatives (12) and (13b,c), respectively.1).2-Cyano-N-(4-(1-methyl-1H-benzo[d]-imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-2-(4-oxo-3-phenylthiazolidin-2-ylidene)acetamide (12).This compound was obtained as yellow powder (DMF), (65%, yield), mp 266-268°C; IR υmax / cm-1 (KBr) 3347 (NH), 2223 (CN), 1685 (CO), 1648 (CO), 1630 (C=N); 1H NMR (DMSO-d6) δ 2.69 (s, 3H, SCH3), 3.73(s, 2H, CH2), 3.89 (s, 3H, NCH3), 7.38-7.97 (m, 14H, Ar-H), 10.31 (s, 1H, NH); m/z 577 (M+, 19.27). Anal. Calcd. For C30H23N7O2S2 (577.68): C, 62.37; H, 4.01; N, 16.97; S, 11.16%. Found:. C, 62.34; H, 3.99; N, 16.87; S, 11.10%2).2-Cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-2-(4-methyl-3- phenylthiazol-2(3H)-ylidene)acetamide (13b).This compound was obtained as pale brown powder (DMF/EtOH), (46%, yield), mp >300°C; IR υmax / cm-1 (KBr) 3218 (NH), 2204 (CN), 1663 (CO), 1630 (C=N); 1H NMR (DMSO-d6) δ 1.72 (s, 3H, CH3), 2.70 (s, 3H, SCH3), 3.89 (s, 3H, NCH3), 6.93 (s, 1H, =CH), 7.27-7.92 (m, 14H, Ar-H), 10.53 (s, 1H, NH); m/z 575 (M+, 19.27). Anal. Calcd. For C31H25N7OS2 (575.71): C, 64.67; H, 4.38; N, 17.03; S, 11.14%. Found: C, 62.61; H, 4.36; N, 17.10; S, 11.11%.3).2-(4-(Benzo[d]thiazol-2-yl)-3-phenylthiazol-2(3H)-ylidene-2-cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)acetamide (13c).This compound was obtained as dark brown crystals (DMF), (61%, yield), mp >300°C; IR υmax / cm-1 (KBr) 3243 (NH), 2214 (CN), 1665 (CO), 1632 (C=N); 1H NMR (DMSO-d6) δ 2.70 (s, 3H, SCH3), 3.88 (s, 3H, NCH3), 6.89 (s, 1H, =CH), 7.42-8.21 (m, 18H, Ar-H), 10.49 (s, 1H, NH); m/z 694 (M+, 19.27). Anal. Calcd. For C37H26N8OS3 (694.85): C, 63.96; H, 3.77; N, 16.13; S, 13.84%. Found:. C, 63.97; H, 3.78; N, 16.11; S, 13.76%.
3.3. General Procedure for the synthesis of 3,5-Diamino-4-cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)thiophene-2-carbo-xamide(18a) and Ethyl 5-(4-(1-methyl-1H-benzo[d]-imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-ylcarbamoyl)-2,4-diaminothiophene-3-carboxylate (18b)
- To a solution of compound (4) (0.01 mol) in 1,4-dioxane (30 ml) containing triethylamine (1 ml), either malononitrile (0.01 mol) or ethyl cyanoacetate (0.01 mol) was added followed by the addition of an equimolar amount of elemental sulfur (0.01 mol). The reaction mixture was heated under reflux for 5h., then cooled and neutralized by pouring onto ice/water mixture containing few drops of hydrochloric acid. The solid product formed in each case was collected by filtration and crystallized from dimethylformamide.1).3,5-Diamino-4-cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)thiophene-2-carboxamide (18a).Dark brown crystals, (60%, yield), mp >300°C; IR υmax / cm-1 (KBr) 3316-3218 (NH and NH2), 2198 (CN), 1662 (CO), 1625 (C=N); 1H NMR (DMSO-d6) δ 2.71 (s, 3H, SCH3), 3.91 (s, 3H, NCH3), 4.42 (2s, 2H, NH2), 4.56 (2s, 2H, NH2), 7.22-7.98 (m, 9H, Ar-H), 10.21 (s, 1H, NH); m/z 500 (M+, 13.34). Anal. Calcd. For C24H20N8OS2 (500.6): C, 57.58; H, 4.03; N, 22.38; S, 12.81%. Found: C, 57.56; H, 4.05; N, 22.36; S, 12.79 %.2).Ethyl 5-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3- (methylthio)-1-phenyl-1H-pyr-azol-5-ylcarbamoyl)-2,4-diaminothiophene-3-carboxylate (18b).Brown crystals, (56%, yield), mp >300°C; IR υmax / cm-1 (KBr) 3436-3385(NH2), 3256 (NH), 2205 (CN), 1696, 1650 (2CO), 1625 (C=N); 1H NMR (DMSO-d6) δ 1.12 (t, J = 7.00 Hz, 3H, CH3), 2.70 (s, 3H, SCH3), 3.49 (q, J = 7.00 Hz, 2H, CH2), 3.90(s, 3H, NCH3), 3.98 (s, 2H, NH2), 4.15 (s, 2H, NH2), 7.23-7.96 (m, 9H, Ar-H), 10.23 (s, 1H, NH); m/z 547 (M+, 12.11). Anal. Calcd. For C26H25N7O3S2 (547.65): C, 57.02; H, 4.60; N, 17.90; S, 11.71%. Found: C, 57.10; H, 4.57; N, 17.88; S, 11.75%.3).4-Amino-2,3-dihydro-N-(4-(1-methyl-1H-benzo [d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-3-phenyl-2-thioxothiazole-5-carboxamide (20)To a solution of compound (4) (0.01 mol) in absolute ethanol (30 ml) containing triethylamine (1 ml) and elemental sulfur (0.01 mol) was added followed by the addition of an equimolar amount of phenyl isothiocyanate (0.01 mol). The reaction mixture was heated under reflux for 1h., at 75°C with continuous stirring and then cooled and neutralized by pouring onto ice/water mixture containing few drops of hydrochloric acid. The solid product formed was collected by filtration and crystallized from dimethylformamide and ethanol.White powder, (52%, yield), mp 295-297°C; IR υmax / cm-1 (KBr) 3421-3382 (NH2), 3250 (NH), 2208 (CN), 1650 (CO), 1628 (C=N); 1H NMR (DMSO-d6) δ 2.70 (s, 3H, SCH3), 5.11 (s, 2H, NH2), 3.90 (s, 3H, NCH3), 7.21-7.92 (m, 14H, Ar-H), 10.19(s, 1H, NH); m/z 569 (M+, 53.11). Anal. Calcd. For C28H23N7OS3 (569.72): C, 59.03; H, 4.07; N, 17.21; S, 16.88%. Found: C, 59.10; H, 4.10; N, 17.18; S, 16.79%.4).2-Cyano-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-3-phenylacrylamide (21).To a solution of compound (4) (0.01 mol) in 1,4-dioxane (30 ml) containing piperidine (0.5 ml), benzaldehyde (0.01 mol) was added. The reaction mixture was heated under reflux for 3h., then left to cool. The solid product so formed was collected by filtration and crystallized from ethanol.Pale yellow crystals, (46%, yield), mp 215-217°C; IR υmax / cm-1 (KBr) 3250 (NH), 2221 (CN), 1679 (CO), 1637 (C=N); 1H NMR (DMSO-d6) δ 2.71 (s, 3H, SCH3), 3.90 (s, 3H, NCH3), 6.25 (s, 1H, CH=C), 7.21-7.91 (m, 14H, Ar-H), 10.28 (s, 1H, NH); m/z 490 (M+, 27.32). Anal. Calcd. For C28H22N6OS (490.58): C, 68.55; H, 4.52;N, 17.13; S, 6.54%. Found: C, 68.53; H, 4.49; N, 17.11; S, 6.56%.
3.4.General Procedure for the synthesis of 2-(4-(1-Methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-ylamino)-6-amino-4-phenyl-4H-pyran-3,5-dic-arbonitrile (22a) and Ethyl 6-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methyl-lthio)-1-phenyl-1H-pyrazol-5-ylamino)-2-amino-5- cyano-4-phenyl-4H-pyran-3-carboxylate (22b)
- To a solution of compound (21) (0.01 mol) in 1,4-dioxane (30 ml) containing triethylamine (0.5 ml), either malononitrile (0.01 mol) or ethyl cyanoacetate (0.01 mol) was added. The reaction mixture was heated in each case under reflux for 6h., then the excess solvent was evaporated under reduced pressure. The solid product formed in each case was collected by filtration and crystallized from dimethylformamide/ethanol (2:1).1).2-(4-(1-Methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-ylamino)-6-amino-4-phenyl-4H-pyran-3,5-dicarbonitrile (22a).White crystals, (52%, yield), mp 267-269°C; IR υmax / cm-1 (KBr) 3345 (NH2), 3255 (NH), 2222, 2219 (2CN), 1650 (CO), 1635 (C=N); 1H NMR (DMSO-d6) δ 2.70 (s, 3H, SCH3), 3.90 (s, 3H, NCH3), 4.76 (s, 2H, NH2), 6.35 (s, 1H, pyran H-4), 7.21-7.84 (m, 14H, Ar-H), 10.23 (s, 1H, NH); m/z 556 (M+, 53.11). Anal. Calcd. For C31H24N8S (556.64): C, 66.89; H, 4.35; N, 20.13; S, 5.76%. Found: C, 66.86; H, 4.31; N, 20.12; S, 5.75%.2).Ethyl 6-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3 -(methylthio)-1-phenyl-1H-pyra-zol-5-ylamino)-2-amino-5-cyano-4-phenyl-4H-pyran-3-carboxylate (22b).Pale yellow crystals, (51%, yield), mp 201-203°C; IR υmax / cm-1 (KBr) 3368(NH2), 3259 (NH), 2222 (CN), 1698, 1652 (2CO), 1629 (C=N); 1H NMR (DMSO-d6) δ 1.33(t, 3H,J = 6.78 Hz, CH3), 2.72 (s, 3H, SCH3), 3.90 (s, 3H, NCH3), 4.24 (q, 2H, J = 6.78 Hz, CH2), 4.83 (s, 2H, NH2), 6.62 (s, 1H, pyran H-4), 7.31-7.81 (m, 14H, Ar-H), 10.21 (s, 1H, NH); m/z 603 (M+, 32.54). Anal. Calcd. For C33H29N7O3S (603.69): C, 66.65; H, 4.84; N, 16.24; S, 5.31%. Found: C, 66.63; H, 4.82; N, 16.19; S, 5.30%.
3.5. General Procedure for the synthesis of 3-Amino-N-(4-(1-methyl-1H-benzo-[d]-imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-5-phenyl-1H-pyrazole-4-carboxamide(25a), 3-Amino-N-(4-(1-methyl-1H-benzo[d]imid-azol-2-yl)-3-(methyl-thio)-1-phenyl-1H-pyrazol-5-yl)-1,5-diphenyl-1H-pyrazole-4-carboxamimide (25b) and N-(4-benzylidene-4,5-dihydro-5-amino-1H-pyrazol-3-yl)-4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-amine (26)
- To a solution of compound (21) (0.01 mol) in 1,4-dioxane (25 ml) and dimethylformamide (5 ml), either hydrazine hydrate (0.01 mol) or phenyl hydrazine (0.01 mol) was added. The reaction mixture in each case was heated under reflux for 6h. The solid products formed, were collected by filtration, and crystallized from dimethylformamide and 1,4-dioxane mixture. The mother liquor in case of hydrazine hydrate pouring onto ice/water mixture containing few drops of hydrochloric acid. The solid products formed, were collected by filtration, and crystallized from dimethylformamide.1).3-Amino-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-5-phenyl-1H-pyrazole-4-carboxamide (25a).Pale brown crystals, (51%, yield), mp 241-243°C; IR υmax / cm-1 (KBr) 3442, 3259 (2NH and NH2), 1645 (CO), 1629 (C=N); 1H NMR (DMSO-d6) δ 2.72 (s, 3H, SCH3), 3.90 (s, 3H, NCH3), 4.23 (s, 2H, NH2), 7.31-7.83 (m, 14H, Ar-H), 10.21 (s, 1H, NH); 13.10 (s, 1H, NH); m/z 520 (M+, 16.56). Anal. Calcd. For C28H24N8OS (520.61): C, 64.60; H, 4.65; N, 21.52; S, 6.16%. Found: C, 64.58; H, 4.61; N, 21.48; S, 6.13%.2).3-Amino-N-(4-(1-methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-yl)-1,5-diphenyl-1H-pyrazole-4-carboxamide (25b)Pale orange crystals, (48%, yield), mp 297-300°C; IR υmax / cm-1 (KBr) 3368 (NH2), 3262 (NH), 1648 (CO), 1625 (C=N); 1H NMR (DMSO-d6) δ 2.70 (s, 3H, SCH3), 3.89 (s, 3H, NCH3), 4.11 (s, 2H, NH2), 7.31-7.81 (m, 19H, Ar-H), 10.29 (s, 1H, NH); m/z 596 (M+, 19.83). Anal. Calcd. For C34H28N8OS (596.7): C, 68.44; H, 4.73; N, 18.78; S, 5.30%. Found: C, 68.42; H, 4.70; N, 18.77; S, 5.21%.3).N-(4-Benzylidene-4,5-dihydro-5-amino-1H-pyrazol-3-yl)-4-(1-methyl-1H-benzo-[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-amine (26)yellow crystals, (13%, yield), mp 129-131℃; IR υmax / cm-1 (KBr) 3248 (NH), 1629 (C=N). m/z 504 (M+, 32.54). Anal. Calcd. For C28H24N8S (504.61): C, 66.65; H, 4.79; N, 22.21; S, 6.35%. Found: C, 66.61; H, 4.78; N, 22.19; S, 6.36%.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML