-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(6): 151-160
doi: 10.5923/j.ajoc.20120206.05
A Facile Synthesis of New 3-(1-Methyl benzimidazol-2-yl) Pyrazolopyrimidine and Pyrimido[2, 1-b][1,3]benzothiazole Derivatives of Potential Biosignificant Interest
Samia M. Sayed , Mohamed. A. Khalil , Mohamed A. Raslan
Chemistry Department, Faculty of Science, Aswan University, 81528 Aswan, Egypt
Correspondence to: Mohamed A. Raslan , Chemistry Department, Faculty of Science, Aswan University, 81528 Aswan, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A simple route for the synthesis of pyrazolo[1,5-a]pyrimidine derivatives was described through the reaction of readily accessible 4-(1-methyl benzimidazol-2-yl)-3-(methylthio)-1H-pyrazol-5-amine (3) with different reagents is described. Also 2-amino benzothiazole in N, N/-dimethyl formamide and anhydrous potassium carbonate reacted with 2-(1-methyl benzimidazol-2-yl)-3,3-bis(methylthio)acrylonitrile (2) to yield pyrimido[2,1-b]benzo-thiazole (21). The latter was further reacted with selected N-, O- and C- nucleophiles such as aryl amines, hetaryl amines, substituted phenols and compounds with an active methylene group. The in vitro antimicrobial activity of some synthesized compounds was examined. Most of the tested compounds proved to be active as antibacterial and antifungal agents.
Keywords: 5-Aminopyrazole, Active Methylene and β-Arylacrylonitrile
Cite this paper: Samia M. Sayed , Mohamed. A. Khalil , Mohamed A. Raslan , "A Facile Synthesis of New 3-(1-Methyl benzimidazol-2-yl) Pyrazolopyrimidine and Pyrimido[2, 1-b][1,3]benzothiazole Derivatives of Potential Biosignificant Interest", American Journal of Organic Chemistry, Vol. 2 No. 6, 2012, pp. 151-160. doi: 10.5923/j.ajoc.20120206.05.
Article Outline
1. Introduction
- Pyrazolopyrimidine derivatives and relatedheteroaromatics are found to possess wide applications in medicine and agriculture. They are biologically interesting isomeric purine analogues and have importance properties as antimetabolites in purine biochemical reaction[1-3]. They exhibited diversified pharmacological activities like antitumor[4], antileukemio[5], tuberculostatic[6], antimicrobial activities [7], neuroleptic[8], CNS depressant[9], antihypertensive[10] and antileishmanial[11].On the other hand, benzimidazole has been also found wide medicinal application as potent antihypertensive[12], antihistaminic[13], anticancer[14, 15] and anti-inflammatory [16] agents, as gastric Ulcer inhibitors[17] and for treatment of cardiovascular diseases[18]. In addition, several benzimidazole derivatives are useful in the textile industry as dying agents[19, 20]. Moreover, the pyrazole ring has shown to be the basic moiety for a number of dyes, drugs and agrochemicals[21-25]. As a part of our programmed[26, 27] amid at the synthesis of novel benzimidazole derivatives which could be useful for biological and pharmacological screening, we aimed to incorporate the benzimidazole moiety into the 4-position of pyrazolo[1,5-a]- pyrimidine ring system to thus obtain a new heterocyclic system which is expected to possess notable chemical and pharmacological activities. Also pyrimidine and iminopyrimidine and fused benzothiazole heterocycles are reported to be effective pharmacophores. Biological activities and various applications of benzothiazole compounds and compounds containing pyrimidine ring have stimulated considerable interest to explore the synthesis of new potential compounds in which pyrimidine ring is fused with another biologically active nucleus such as benzothiazole and benzimidazole through nitrogen atom. Very few references are available on the synthesis of pyrimido benzothiazole compounds. The synthesis of acidic derivatives of 4H-pyrimido[2,1-b]benzazole-4-ones by the condensation of 2-aminobenzo-thiazole, 2-amino benzooxazole and 2-amino-1-methylbenzimidazole independently with 2-aminofumarate and diethyl ethoxy methylene malonate and their anti-allergic activity were reported require the presence of steam of nitrogen gas, hance it was considered appropriate to devise a convenient route to synthesis of the these compounds. It is surmised that pyrimido[2,1-a][1,3]benzothiazole incorporatingbenzimidzole nucleus would exhibit interesting properties and pharmacological[28 and references cited in].
2. Results and Discussion
2.1. Chemistry
- Thus, it has been found that treatment of 2-(1-methyl benzimidazol-2-yl) 3,3-bis-(methylthio)acrylonitrile (2) with hydrazine hydrate and phenylhydrazine affords the target 5-aminopyrazole (3) and (4), respectively. The structure of (3) was established and confirmed as the reaction product on the basis of their elemental analysis and spectral data (MS, IR and 1H NMR and 13C NMR). The analytical data for (3) revealed a molecular formula C12H13N5S (M+ = 259 m/z). The IR spectrum showed absorption bands in the region 3380 and 3230cm-1 for NH and NH2, in addition to disappearance of cyano function signal. The 1H NMR of (3) revealed a bands at δ= 2.64, 3.89, 5.85 and 13.2 ppm assignable to a SCH3, N-CH3, NH2 and NH-pyrazole; respectively. (cf. Scheme 1)
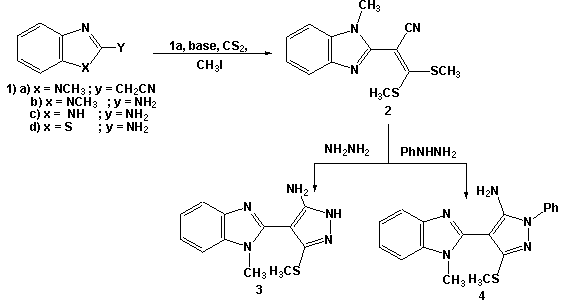 | Scheme 1. Synthesis of 5-aminopyrazzole derivative 3 and 4 |
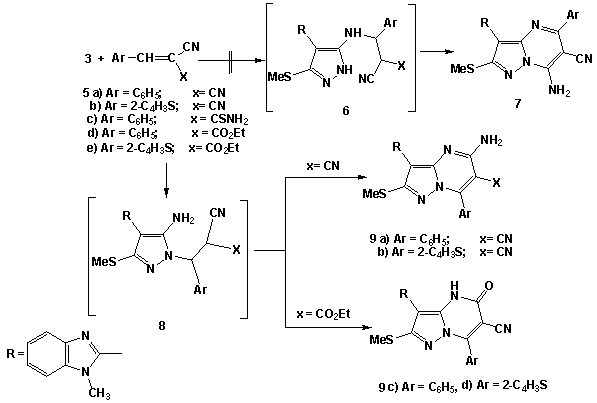 | Scheme 2. Synthesis of pyrazolo[1,5-a]pyrimidine dervatives 9a-d |
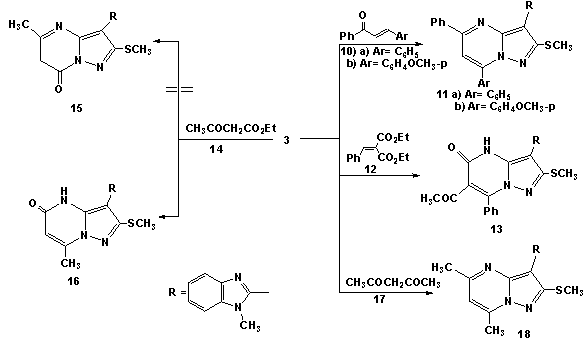 | Schem 3. Reaction of 5-aminopyrazole (3) with chalcones (10a,b) and active methylene (14) and (17) |
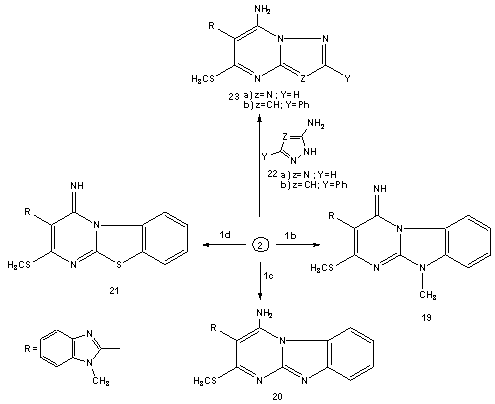 | Schem 4. Synthesis of pyrimidobenzimidazole (19,20), pyrimidobemzothiazole (21) and triazolopyrimidine (23a) derivatives |
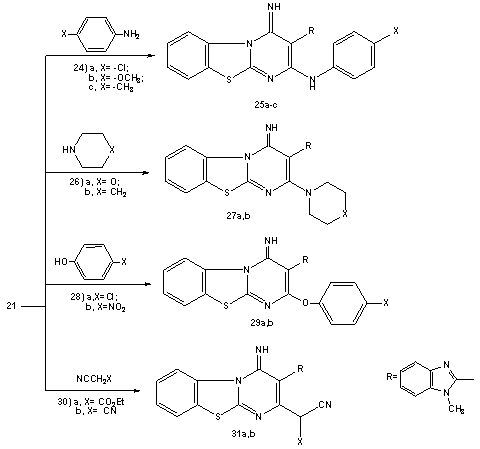 | Scheme 5. Reaction of pyrimidobenzothiazole derivative (21) with aniline ; phenol and cyanomethlylene derivatives |
2.2. Antimicrobial Activity
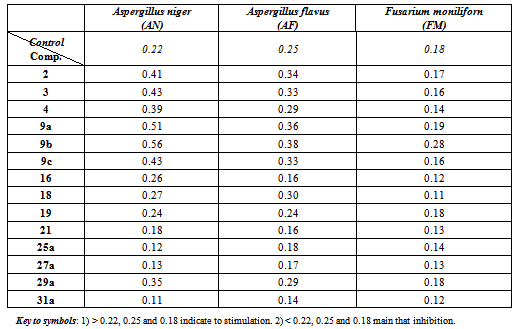
- The newly synthesized products 2, 4, 9a-c, 16, 18, 19, 21, 25a, 27a, 29a and 31 were tested for their antimicrobial activities using three species of fungi, namely Aspergillus niger AN, Aspergillus flavus AF and Fusarium moniliform FM. The organisms were grown on a liquid schapex,s media supplemented with the tested compounds and the growth were measured using the dry weight method[35].
2.2.1. Discussion of Stimulation
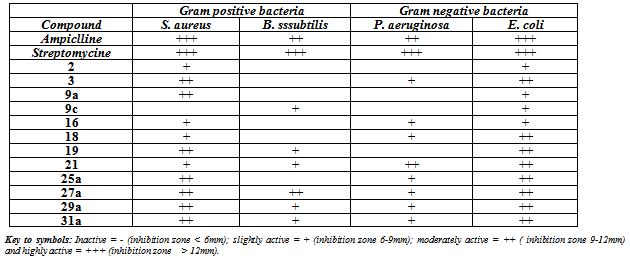
- Aspergillus has a large chemical repertoire. Commodity products produced in Aspergillus cell "factories" include citric, gluconic, itaconic and kojic acid. The production of citric acid by using of Aspergillus niger back to Currie[36]. Citric acid is one of the most widely used food ingredients. It also has found use in the pharmaceutical and cosmetic industries as an acidulant and for aiding in the dissolution of active ingredients. Other technical applications of citric acid are as a hardener in adhesive and for retarding the setting of concrete[37]. Citric acid is a true bulk chemical with an estimated production approximating more than 1.6 billion kg each year[38]. Aspergillus niger also has found use in the industrial production of gluconic acid, which is used as an additive in certain metal cleaning applications, as well as for the therapy for calcium and iron deficiencies[39].Also the newly synthesized products 2, 4, 9a-c, 16, 18, 19, 21, 25a, 27a, 29a and 31 were investigated against four pathogenic representative microorganism Staphylococcus aureus and Bacillus subtilis (gram positive bacteria) and Psuedomonas aeruginosa and Escherichia coli (gram negative bacteria) using Ampicillin and streptomycine as standard drugs. Agar well-diffusion method[40] was used for studying the potential activities of these compounds. As shown in Table 2. the antimicrobial effect of the tested compounds was evaluated by measuring the zone diametera and their results were compared with those of well known drugs (standards).
|
|
3. Experimental
- Melting points were measured on a Gallenkamp apparatus and are uncorrected. IR spectra were recorded on Shimadzu FT-IR 8101 PC infrared spectrophotometer. The 1H NMR and 13C NMR spectra were determined in DMSO-d6 at 300 MHz on a Varian Mercury VX 300 NMR spectrometer using TMS as an internal standard. Mass spectra were measured on a GCMS-QP1000 EX spectrometer at 70Ev. Elemental analyses were carried out at the Microanalytical Center of Cairo University.2-(1-Methylbenzimidazol-2-yl)-3,3-bis(methylthio)acrylonitrile (2)To solution of sodium hydride (0.45 mole) in benzene (150ml), a solution of 1-methyl benzimidazol-2-yl acetonitrile (1a) (0.2 mole), carbon disulphide (0.2 mole) in dry dimethylformamide (100 ml) was added in portions during 1 hr. The reaction mixture was kept under stirring for 3hrs. followed by addition of methyliodide (0.4 mole) in portions with cooling. The reaction mixture was allowed to stand at room temperature for 5h. and then refluxed for addition 4h. After cooling, it was poured onto ice/water, the resulting solid was collected by filtration and finally recrystallisted from DMF to afford (72% yield) of compound 2. mp 220°C; IR υmax / cm-1 (KBr) 2176 (CN), 1615 (C=N); 1H NMR (DMSO-d6) δ= 2.58(s, 3H, SCH3), 2.65(s, 3H, SCH3), 3.9(s, 3H, NCH3), 7.15-7.78(m,4H, Ar-H). 13C-NMR 17.3, 31.2(SCH3, NCH3), 118.2(CN), 122, 116.3, 132.6, 138.5, 141.2(benzimidazole carbons), 83, 171.1(C=C) M/S: m/z 275(M+, 62.8. (Found: C, 56.63, H; 4.65; N, 15.12; S, 23.43. C13H13N3S2 require C, 56.70; H, 4.76; N, 15.26; S, 23.28 %)4-(1-Methylbenzimidazol-2-yl)-3-(methylthio)-1H-pyrazol-5-amine (3)A solution of 2-(1-methyl benzimidazol-2-yl) 3,3-bis(methylthio)acrylonitrile 2 (0.1 mol) in absolute ethanol (150ml) was treated with hydrazine hydrate (0.12 mol). The reaction mixture was refluxed for about 4-6h. (TLC control). After evaporation of the solvent, the solid product was collected by filtration and recrystallised from ethanol/DMF to afford (79%, yield) of 3, mp 260°C; IR υmax / cm-1 (KBr) 3275, 3172 (NH and NH2), 1627(C=N); 1H NMR (DMSO-d6) δ= 2.64(s, 3H, SCH3), 3.89(s, 3H, NCH3) 5.85(br, 2H, D2O exchangeable NH2), 7.35-7.76(m,4H, Ar-H), 13.2(s, 1H, NH). 13C-NMR 14.8, 31.9(SCH3, NCH3), 122, 116.3, 132.6, 138.5, 152.2(benzimidazole C), 104, 139.1, 150.2(pyrazole C) M/S: m/z 259(M+, 86.19),. (Found: C, 55.32;H, 4.1; N, 27.2; S, 12.24. C12H13N5S. requires C, 55.58; H, 5.05; N, 27.01; S, 12.36%).4-(1-Methyl-1H-benzo[d]imidazol-2-yl)-3-(methylthio)-1-phenyl-1H-pyrazol-5-amine (4)A mixture of 2 (0.02 mol), phenylhydrazine (0.02 mol) and 0.1 ml of triethylamine was refluxed in 50 ml of absolute ethanol for 3-5h (TLC control). The solvent was evaporated in vacuo. The remaining solid was triturated with methanol. The solid product was collected by filtration and finally recrystallization from ethanol/DMF solvent to afford (68%, yield) of compound 4, mp. 270-271°C; IR υmax/cm-1 (KBr) 3430, 3285, (NH2), 1635(C=N); 1H NMR (DMSO-d6) δ= 2.62(s, 3H, SCH3), 3.98(s, 3H, NCH3) 5.72(br, 2H, D2O exchangeable NH2), 7.31-7.75(m, 8H, Ar-H). (Found: C, 64.41; H, 5.13; N, 20.82; S, 9.52. C18H17N5S requires C, 64.45; H, 5.11; N, 20.88; S, 9.56%).Reaction of 5-aminopyrazole (3) with cinnamonitriles (5a-e), chalcones (10a,b) and α-acetyl ethylcinnamate (12).General procedureA suspension of 3 (0.01 mol) and the appropriate cinnamonitriles 5a-e, chalcones (10a,b) and/or α-acetyl ethylcinnamate (12) (0.01 mol) in absolute ethanol was treated with piperidine (1 ml). The reaction mixture was refluxed for 3-5h. (TLC control), the solvent was then evaporated in vacuo. The remaining solid was triturated with ice water and acidified with concentrated HCl. The product was collected by filteration and finally recrystallised from the appropriate solvent to afford the corresponding 5-aminpyrazolo[1,5-a]pyrimidine derivatives (9a-d), (11a,b) and (13), respectively. 2-Amino-8-(1-methylbenzimidazol-2-yl)-7-(methylthio)-4-phenylpyrazolo[1,5-a]-pyrimidine-3-carbonitrile (9a): Yield, 70%; mp. 345°C, IR υmax / cm-1 (KBr) 3337, 3285(NH2), 2208(CN), 1631(C=N); 1H NMR (DMSO-d6) δ=2.63(s, 3H, SCH3), 3.97(s, 3H, NCH3) 4.1(br, 2H, D2O exchangeable NH2), 7.31-7.75(m, 9H, Ar-H). 13C-NMR 116.9(CN); 14.8, 31.7(SCH3, NCH3), 123, 115.8, 134.6, 138.7, 153.2-(benzimidazole C), 87, 166.4, 168.4 (pyrimidine C); 104, 136.1, 135.2(pyrazole C), 126.2, 129.3, 128.1, 136.2 (C6H5 C). M/S: m/z 411(M+, 18.4). (Found: C, 64.2; H, 4.11; N, 23.77; S, 7.67.C22H17N7S requires C, 64.22; H, 4.17; N, 23.83; S, 7.79%).2-Amino-8-(1-methylbenzimidazol-2-yl)-7-(methylthio)-4-(thieno-2-yl)pyrazolo-[1,5-a]pyrimidine-3-carbonitrile (9b): Yield, 68%; mp. 350°C, IR υmax / cm-1 (KBr) 3263, 3203(NH2), 2191(CN), 1628(C=N); 1H NMR (DMSO-d6) δ=2.63(s, 3H, SCH3), 3.97(s, 3H, NCH3) 4(brs, 2H, D2O exchangeable NH2), 7.31-7.75(m, 7H, Ar-H). (Found: C, 57.45; H, 3.35; N, 23.43; S, 15.36.C20H15N7S2 requires C, 57.54; H, 3.62; N, 23.48; S, 15.36%).3-cyano-8-(1-methylbenzimidazol-2-yl)-7-(methylthio)-4-phenylpyra-zolo[1,5-a]-pyrimidine-2-(1H)-one (9c): Yield, 63%; mp. 280°C, IR υmax / cm-1 (KBr) 3333, 3170(NH), 2196(CN), 1668(C=O), 1631(C=N); 1H NMR (DMSO-d6) δ=2.64(s, 3H, SCH3), 3.98(s, 3H, NCH3) 5.91(s, 1H, D2O exchangeable NH), 7.31-7.75(m, 9H, Ar-H); M/S: m/z 413(M++1, 5.9), 412(M+, 32.3). (Found: C, 66.10; H, 3.82; N, 20.23; S, 7.71. C22H16N6SO requires C, 66.06; H, 3.91; N, 20.37; S, 7.77%).3-cyano-8-(1-methylbenzimidazol-2-yl)-7-(methylthio)-4-(thieno-2-yl)-pyrazolo-[1,5-a]pyrimidine-2-(1H)-one (9d): Yield, 68%; mp. 180°C, IR υmax / cm-1 (KBr) 3343, 3274 (NH), 2186(CN), 1658(C=O), 1628(C=N); 1H NMR (DMSO-d6) δ= 2.63(s, 3H, SCH3), 3.99(s, 3H, NCH3) 6.1(s, 1H, D2O exchangeable NH), 7.31-7.75(m, 7H, Ar-H). (Found: C, 57.31; H, 3.34; N, 20.10; S, 15.21. C20H14N6S2O requires C, 57.40; H, 3.37; N, 20.08; S, 15.32%).8-(1-methylbenzimidazol-2-yl)-7-(methylthio)-2,4-diphenylpyrazolo[1,5-a]pyr-imidine (11a): Yield, 62%; mp. 215°C, IR υmax / cm-1 (KBr), 1635(C=N); 1H NMR (DMSO-d6) δ=2.63(s, 3H, SCH3), 3.99(s, 3H, NCH3), 7.19-7.97(m, 14H, Ar-H) 8.38(s, 1H, pyrimidine), M/S: m/z 448(M++1, 8.9), 447(M+, 63.1). (Found: C, 72.48; H, 4.65; N, 15.67; S, 7.12. C27H21N5S requires C, 72.46; H, 4.73; N, 15.65; S, 7.16%).8-(1methylbenzimidazol-2-yl)-4-(4-methoxyphenyl)-7-(methylthio)-2-phenylpyr-azolo[1,5-a]pyrimidine (11b): Yield, 58%; mp. 238°C, IR υmax / cm-1 (KBr), 1635(C=N); 1H NMR (DMSO-d6) δ =2.6(s, 3H, SCH3), 3.97(s, 3H, NCH3) 4.1(s, 3H, CH3), 7.31-7.98(m, 13H, Ar-H), 8.35(s, 1H, pyrimidine). (Found: C, 70.52; H, 4.81; N, 14.66; S, 6.74. C28H23N5SO requires C, 70.42; H, 4.85; N, 14.67; S, 6.71%).3-Acetyl-8-(1methylbenzimidazol-2-yl)-7-(methylthio)-4-phenylpyrazolo[1,5-a]-pyrimidin-2(1H)-one (13): Yield, 53%; mp. 264°C, IR υmax / cm-1 (KBr), 1685, 1660(C=O), 1635(C=N); 1H NMR (DMSO-d6) δ= 2.51(s, 3H, COCH3), 2.62(s, 3H, SCH3), 3.97(s, 3H, NCH3), 7.23-8.08(9H, m), 8.45(s, 1H, pyrimidine), M/S: m/z 429(M+, 53.2). (Found: C, 64.31, H, 4.50; N, 16.23; S, 7.45. C23H19N5SO2 requires C, 64.32; H, 4.46; N, 16.31, S, 7.46%).8-(1-methylbenzimidazol-2-yl)-4-methyl-7-(methylthio) pyrazolo[1,5-a]pyrimi-din-2-(1H)-one (16) and 8-(1-methylbenzimidazol-2-yl)-2,4-dimethyl)-7-(methyl-thio) pyrazolo[1,5-a]pyrimidine (18).General procedureA solution of 3 (0.01mol) in acetic acide (30 ml)was treated with ethyl acetoacetate (14) and acetylacetone (17) (0.01 mol). The solution was refluxed for 3h and the obtained product was recrystallized from ethanol to give 16 and 18, respectively.Compound (16): Yield, 67%; mp. 270°C, IR υmax / cm-1 (KBr) 3500, 3330(NH), 1660(C=O) 1635(C=N); 1H NMR (DMSO-d6) δ = 2.35(s, 3H, CH3), 2.61(s, 3H, SCH3), 3.98(s, 3H, NCH3) 6.1(s, 1H, D2O exchangeable NH), 7.31-7.85(m, 4H, Ar-H), 8.76(s, 1H, pyrimidine), M/S: m/z 325(M+, 83.3). (Found: C, 59.12; H, 4.62; N, 21.51; S, 9.89. C16H15N5SO requires C, 59.06; H, 4.65; N, 21.52; S, 9.85%).Compound (18): Yield, 53%; mp. 280°C, IR υmax / cm-1 (KBr) 1635(C=N); 1H NMR (DMSO-d6) δ= 2.1(s, 3H, CH3), 2.35(s, 3H, CH3), 2.61(s, 3H, SCH3), 3.98(s, 3H, NCH3), 7.23-7.95(m, 4H, Ar-H), 8.46(s, 1H, pyrimidine). (Found: C, 63.13; H, 5.28; N, 21.66; S, 9.89. C17H17N5S requires C, 63.13; H, 5.3; N, 21.66; S, 9.91%).4-Imino-2-methylthio-3-(1-methylbenzimidazol-2-yl)-4H-pyrimido[1,2-a]benz-imidazole (19);4-Amino-2-methylthio-3-(1-methylbenzimidazol-2-yl)-4H-pyr-imido[1,2-a]benzimidazole (20);4-Imino-2-methylthio-3-(1-methyl benzimidazol-2-yl)-4H-pyrimido[2,1-b]benzothiazole (21) 4-Amino-2-methylthio-3-(1-methylbenzimidazol-2-yl)-1,2,4-triazolo[2,3-a]pyrimidine (23a) and4-Amino-2-methyl-thio-3-(1-methyl benzimidazol-2-yl)-7-phenyl pyrazolo[1,5-a]pyrimidine (23b), respectively.General procedureA suspension of (2) (0.01 mol) and the appropriate 2-amino benzimidazole derivatives (1b,c) and 2-amino benzothiazole (1d), 5-amino-1H-1,2,4-triazole (22a) and 5-amino-3-phenyl-1H-pyrazole (22b) (0.01 mol) in 15 ml of N, N/-dimethyl formamide and anhydrous potassium carbonate (10mg) was refluxed for 4-6 hours. The reaction mixture was refluxed for 3-5h. (TLC control), the solvent was then evaporated in vacuo. The remaining solid was coold to room temperature and triturated with ice cold water. The product was collected by filtration, washed with water several times and finally recrystallized from the appropriate solvent to afford the corresponding pure (19-21) and (23a,b) derivatives, respectivelyCompound (19): Yield, 67%; mp. 282°C, IR υmax / cm-1 (KBr) 3400, 3335(NH), (C=N); 1H NMR (DMSO-d6) δ =2.65 (s, 3H, SCH3), 3.96(s, 3H, NCH3), 3.98(s, 3H, NCH3), 7.21-7.98(m, 8H, Ar-H), 9.68(s, 1H, D2O exchangeable =NH), M/S: m/z 375(M++1,3.9), 374(M+,72.2). (Found: C, 64.12; H, 4.83; N, 22.51; S, 8.59. C20H18N6S requires C, 64.15; H, 4.84; N, 22.44; S, 8.56%).Compound (20): Yield, 63%; mp. 310°C, IR υmax / cm-1 (KBr) 3320, 3180 (NH2), 1630 (C=N); 1H NMR (DMSO-d6) δ = 2.65 (s, 3H, SCH3), 3.96(s, 3H, NCH3), 7.21-8.19(m, 10H, Ar-H) M/S: m/z 360(M+, 79.6). (Found: C, 63.11; H, 4.45, N, 23.29; S, 8.78. C19H16N6S requires C, 63.31; H, 4.48; N, 23.32; S, 8.89%).Compound (21): Yield, 57%; mp. 256°C, IR υmax / cm-1 (KBr) 3350, 3335(NH), (C=N); 1H NMR (DMSO-d6) δ =2.63 (s, 3H, SCH3), 3.99(s, 3H, NCH3), 7.23-7.96(m, 8H, Ar-H), 9.65(s, 1H, D2O exchangeable =NH). (Found: C, 64.43; H, 4.10; N, 18.62; S, 17. C19H15N5S2 requires C, 60.45; H, 4.01; N, 18.55; S, 16.99%).Compound (23a): Yield, 72%; mp. 270-2°C, IR υmax / cm-1 (KBr) 3218(NH2), 1620(C=N); 1H NMR (DMSO-d6) δ = 2.64 (s, 3H, SCH3), 3.97(s, 3H, NCH3), 7.21-8.12(m, 6H, Ar-H), 8.8(s, 1H, triazole) M/S: m/z 312(M++1, 9.6), 311(M+, 54.2). (Found: C, 54.10; H, 4.12; N, 31.46; S, 10.4. C14H13N7S requires C, 54.01; H, 4.21; N, 31.49; S, 10.30%).Compound (23b): Yield, 58%; mp.298°C, IR υmax / cm-1 (KBr) 3200, 3190 (NH2), 1628 (C=N); 1H NMR (DMSO-d6) δ = 2.6 (s, 3H, SCH3), 3.99(s, 3H, NCH3), 7.23-8.23(m, 12H, Ar-H). (Found: C, 65.23; H, 4.7; N, 21.59; S, 8.23. C21H18N6S requires C, 65.27; H, 4.69; N, 21.75; S, 8.3%).2-Substituted-4-imino-3-(1-methylbenzimidazol-2-yl)-4H-pyrimido[2,1-b][1,3]-benzothiazoles (25a-c; 27a,b; 29a,b and 31a,b).A suspension of (21) (0.01 mol) and independently, the appropriate aromatic amines, hetaryl amines, substituted phenols or compounds containing active methylene group (0.01 mol) in 15 ml of N, N/-dimethyl formamide and anhydrous potassium carbonate (10mg) was refluxed for 4-6 hours. The reaction mixture was refluxed for 3-5h. (TLC control), the solvent was then evaporated in vacuo. The remaining solid was coold to room temperature and triturated with ice cold water. The product was collected by filtration, washed with water several times and finally recrystallized from the appropriate solvent to afford the corresponding pure (25a-c), (27a,b), (29a.b) and (31a,b) derivatives, respectively2-(4/-Chloroanilino)-4-imino-3-(1-methylbenzimidazol-2-yl)-4H-pyrimido[2,1-b]-[1,3]benzothiazole (25a): Yield, 46%; mp.199°C, IR υmax / cm-1 (KBr) 3380, 3335, (NH), 1635 (C=N); 1H NMR (DMSO-d6) δ = 3.99(s, 3H, NCH3), 4.35(brs, 1H, D2O exchangeable NH), 9.97(brs, 1H, D2O exchangeable =NH),7.21-7.98(12H, m), M/S: m/z 457(M++1, 15.5), 456(M+, 48). (Found: C, 63.06; H, 3.71; N, 18.42; S, 7.10, Cl, 7.74. C24H17N6SCl requires C, 63.08; H, 3.75; N, 18.39; S, 7.02, Cl, 7.76%).4-Imino-3-(1-methyl benzimidazol-2-yl)-2-(4/-methoxyanilino)-4H-pyrimido[2,1-b][1,3]benzothiazoles (25b): Yield, 47%; mp.214°C, IR υmax / cm-1 (KBr) 3375, 3330, (NH), 1630 (C=N); 1H NMR (DMSO-d6) δ = 3.71(s, 3H, OCH3), 3.97(s, 3H, NCH3), 4.41(brs, 1H, D2O exchangeable NH), 9.98(brs, 1H, D2O exchangeable =NH),7.21-7.98(12H, m). (Found: C, 66.32; H, 4.47; N, 18.46; S, 7.04. C25H20N6SO requires C, 66.35; H, 4.45; N, 18.52; S, 7.08%).4-Imino-3-(1-methyl benzimidazol-2-yl)-2-(4/-methylanilino)-4H-pyrimido[2,1-b][1,3]benzothiazoles (25c): Yield, 46%; mp.195°C, IR υmax / cm-1 (KBr) 3385, 3340, (NH), 1635 (C=N); 1H NMR (DMSO-d6) δ = 2.34(s, 3H, CH3), 3.98(s, 3H, NCH3), 4.21(brs, 1H, D2O exchangeable NH), 9.98(brs, 1H, D2O exchangeable =NH),7.25-7.86(12H, m). (Found: C, 68.76; H, 4.63; N, 19.32; S, 7.24.C25H20N6S requires C, 68.79; H, 4.62; N, 19.25; S, 7.34%).4-Imino-3-(1-methylbenzimidazol-2-yl)-2-(morpholino)-4H-pyrimido[2,1-b]-[1,3]benzothiazoles (27a): Yield, 44%; mp.208°C, IR υmax / cm-1 (KBr) 3380, 3300, (NH), 1635 (C=N); 1H NMR (DMSO-d6) δ = 3.8(t, 4H, 2 –N-CH2), 4.1(t, 4H, 2–OCH2), 3.99(s, 3H, NCH3), 9.95(brs, 1H, D2O exchangeable =NH),7.25-7.86(8H, m), M/S: m/z 417(M++1, 15.2), 416(M+, 51.2). (Found: C, 63.53; H, 4.84; N, 20.23; S, 7.64.C22H20N6SO requires C, 63.44; H, 4.84; N, 20.18; S, 7.7%).4-Imino-3-(1-methylbenzimidazol-2-yl)-2-(piperidino)-4H-pyrimido[2,1-b][1,3]-benzothiazoles (27b): Yield, 41%; mp.192°C, IR υmax / cm-1 (KBr) 3330, 3310, (NH), 1635 (C=N); 1H NMR (DMSO-d6) δ = 1.6(m, 6H, 3CH2), 2.6(t, 4H, 2NCH2), 3.97(s, 3H, NCH3), 9.92(brs, 1H, D2O exchangeable =NH),7.25-7.86(8H, m). (Found: C, 66.54; H, 5.21; N, 20.18; S, 7.64.C23H22N6S requires C, 66.64; H, 5.35; N, 20.27; S, 7.73%).2-(4/-Chlorophenoxy)-4-imino-3-(1-methylbenzimidazol-2-yl)-4H-pyrimido[2,1-b][1,3]benzothiazole (29a): Yield, 52%; mp.178°C (dec.), IR υmax / cm-1 (KBr) 3314, (NH), 1630 (C=N); 1H NMR (DMSO-d6) δ = 3.98(s, 3H, NCH3), 9.95(brs, 1H, D2O exchangeable=NH),7.25-7.83(12H, m), M/S: m/z 458(M++1, 13.3), 457(M+, 36.3). (Found: C, 62.83; H, 3.54; N, 15.19; S, 6.95, Cl, 7.67. C24H16N5SClO requires C, 62.95; H, 3.52; N, 15.29; S, 7, Cl, 7.74%).4-Imino-3-(1-methylbenzimidazol-2-yl)-2-(4/-nitrophenoxy)-4H-pyrimido[2,1-b]-[1,3]benzothiazoles (29b): Yield, 52%; mp.156°C (dec.), IR υmax / cm-1 (KBr) 3310, (NH), 1633 (C=N); 1H NMR (DMSO-d6) δ = 3.99(s, 3H, NCH3), 9.96(brs, 1H, D2O exchangeable=NH),7.25-7.83(12H, m). (Found: C, 61.51; H, 3.24; N, 17.79; S, 6.74.C24H16N6SO3 requires C, 61.53; H, 3.44; N, 17.94; S, 6.84%).2-(Ethyl cyanoacetyl)-4-imino-3-(1-methylbenzimidazol-2-yl)-4H-pyrimido[2,1-b][1,3]benzothiazole (31a): Yield, 59%; mp.281°C (dec.), IR υmax / cm-1 (KBr) 3380, 3300, (NH), 2203 (CN), 1705 (CO), 1635 (C=N); 1H NMR (DMSO-d6) δ = 1.3(t, 3H, CH3), 3.96(s, 3H, NCH3), 4.01 (s, 1H, CH), 4.14(q, 2H, CH2), 9.93(brs, 1H, D2O exchangeable =NH),7.25-7.86(8H, m), M/S: m/z 442(M+, 47.1). (Found: C, 62.33; H, 4.11; N, 18.91, S, 7.22. C23H18N6SO2 requires C, 62.43; H, 4.1; N, 18.99; S, 7.25%).4-Imino-3-(1-methylbenzimidazol-2-yl)-2-(malononitrile)-4H-pyrimido[2,1-b]-[1,3]benzothiazole (31b): Yield, 62%; mp.243°C (dec.), IR υmax / cm-1 (KBr) 3320, 3305, (NH), 2203, 2198 (CN), 1628 (C=N); 1H NMR (DMSO-d6) δ = 3.96(s, 3H, NCH3), 4.23 (s, 1H, CH), 9.99(brs, 1H, D2O exchangeable =NH),7.25-7.86(8H, m). (Found: C, 63.76; H, 3.33; N, 24.71; S, 8.13. C21H13N7S requires C, 63.78; H, 3.31; N, 24.8; S, 8.11%).
ACKNOWLEDGEMENTS
- The author would like to thank to Aswan University, Unit of Environmental Studies and Development, Aswan, A. R. Egypt, for antimicrobial test results.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML