-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(6): 143-150
doi:10.5923/j.ajoc.20120206.04
Synthesis and Characterization of New 1,3-Oxazol-5-(4H)-one Derivatives
Abdul- Jabbar K. AL. Abodi, Ngam Majed., Sahar A. K., Redha I. H. Al-Bayati
Department of chemistry, college of science AL- Mustansirya University, Baghdad, Iraq
Correspondence to: Abdul- Jabbar K. AL. Abodi, Department of chemistry, college of science AL- Mustansirya University, Baghdad, Iraq.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
New 1,3-oxazol-5(4H)-one (3a – b) was synthesized by cyclization of[(Pyridyl-3-yl-carbonyl)amino]acetic acid (2). The starting were readily obtained by acylation of 2-amino acetic acid (Glycine) with nicotinoyl chloride. Imidazole was synthesized by reaction of compounds (3a - b) with hydrazine hydrate (99%). Compounds (4a - b) were converted into a variety of derivatives. All new compounds were characterized by 1H NMR and FTIR spectroscopy.
Keywords: Nicotinic Acid, 1,3-Oxazole, Triazole, Thiadiazole, Thiourease
Cite this paper: Abdul- Jabbar K. AL. Abodi, Ngam Majed., Sahar A. K., Redha I. H. Al-Bayati, Synthesis and Characterization of New 1,3-Oxazol-5-(4H)-one Derivatives, American Journal of Organic Chemistry, Vol. 2 No. 6, 2012, pp. 143-150. doi: 10.5923/j.ajoc.20120206.04.
Article Outline
1. Introduction
- The synthesis of 1,3-oxazoles have interest due to their various biological activities. Reported some of these activities were: nervous system depression[1, 2], antibacterial[3], herbicidal[4], and muscle relaxant[5] activities. Imidazole is a planer heterocyclic compound with five-member ring has three carbon atoms and two nitrogen atom at 1,3 positions. purine, histamine, histidine and nucleic acid , including imidazole ring[6], Imidazole derivatives possess a broad spectrum of pharmacological activities such as Anti-fungal and Anti-bacterial activity[7]Anticonvulsant[8] anti-Parkinson[9] and monoamine oxidase (MAO) inhibitory[10] activity. Products of Compounds containing a 1H-1,2,4- triazole derivatives and many mono-, di- and tri-substituted 1,2,4-triazole-5-thiones Polyamide containing N-methylimidazole (Im) can be combined in antiparallel side-by-side dimericcomplexes with the minor groove of DNA[11], also compound 2-Amino-3-(4-carboxy-1H-imidazol-1-yl) propionic acid has been examined as an inhibitor of diaminopimelic acid dehydrogenase from Bacillus sphaericus[12]
2. Experimental
2.1. General
- Melting point were determined in open capillary tubes on a Gallen kamp melting point apparatus and are uncorrected. The IR Spectra were recorded by KBr discs using a perkin-Elmer 1600 series FTIR spectrometer. 1HNMR Spectra were recorded on a Varian-Mercury 200 MHZ Spectrometer.
2.2. Synthesis of Nicotinoyl Chloride (1)
- To a solution of nicotinic acid (1.23g, 0.01 mole ) in dry benzene (20 ml), thionyl chloride (1.19g, 0.01 mol) was added. Then, the reaction mixture was refluxed for 7h. After evaporation, the product was collected without recrystallization. (yield 86 %), (m.p, C°) (200-203), IR.(KBr) (ν, Cm-1) 3066 (C-H Ar.), 1799 (C=O, acid chloride) 768 (C-Cl).
2.3. Synthesis of[(Pyridyl-3-yl-Carbonyl Amino] Acetic Acid (2)
- To a stirring solution of glycine (0.75 g, 0.01 mol) and sodium hydroxide (10 ml,10% solution), compound 1 (1.41 g, 0.01 mol) was added. Then, the reaction mixture was shacked vigorously for 1h. ,a few grams of crushed ice was added with stirring. After that, the solution was acidified with conc. HCl and the product was collected and recrystallized from ethanol. (yield 67 %), (m.p, C°)(250-253), ( IR. (KBr) (ν, Cm-1) 3180 (NH), 3178 (acid OH), 2976-2888 (C-H alph.) ,1741 (acid C=O),1690 (amide C=O); 1HNMR (DMSO-d6) ς (ppm) 3.31 (s, NH), 4.42 ( s, CO-CH2-NH), 7.15-8.20 (m, Aromatic protons).
2.4. Synthesis of 4-(Arylidene)-2-(Pyridin-3-yl)-1,3-Oxazol-5(4H)- One(3a- B)
- To a stirring mixture of compound 2 (1.8 g, 0.01 mol) acetic acid (5 ml) acetic anhydride (20 ml), aromatic aldehyde (0.01 mol) was added. The temperature of reaction was reached to 70C° for 10 min. , then the mixture was poured in to crushed ice and stirred for 30 min. the product was collected and recrystallized from ethanol to afforded the desired compound.
2.4.1. (3a). 4-(4-Bromobenzylidene)-2-(Pyridin-3-yl)-1, 3-Oxazol-5(4h)-One
- (yield 71 %), (m.p, C°,120-123), IR. (KBr) (ν, Cm-1) 3050 (C-H ar), 3123 (C-H olifen), 1755 (oxazole C=O), 1656 (oxazole C=N), 1600 - 1511 (C=C Ar), 1280 (C-O) 848 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.90 ( s, C=CH- ), 6.91 – 8.1 (m, Aromatic protons)
2.4.2. (3b). 4-(4-Nitrobenzylidene)-2-(Pyridin-3-yl)-1, 3-Oxazol-5(4h)-One
- (yield 63 %), (m.p, C°, 80-82), IR. (KBr) (ν, Cm-1) 3075 (C-H ar), 3220 (C-H olifen), 1770 (oxazole C=O), 1650 (oxazole C=N), 1600 - 1505 (C=C Ar), 1230 (C-O) 828 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.82 ( s, C=CH- ), 7.21 – 8.1 (m, Aromatic protons)
2.5. Synthesis of 3-Amino-5-(Arylidene)-2-(Pyridin-3-yl)- 3,5-Dihydro-4H-Imidazol-4-One (4a – B)
- To a mixture of compound (3a – b) (0.01 mol) in dry pyridine (5ml) hydrazine hydrate (99%) (10ml) was added. The reaction mixture was refluxed for 20 h. Then, the mixture was allowed to cool to room temperature and pyridine was removed. The product was recrystallized from ethanol to afford the desired compound.
2.5.1. (4a). 3-Amino-5-(4-Bromobenzylidene)-2-(Pyridin-3- yl)-3,5-Dihydro-4h-Imidazol-4-One
- (yield 53 %), (m.p, C°,230-233), IR. (KBr) (ν, Cm-1) 3424-3227 (NH2), 3065 (C-H ar), 3190 (C-H olifen), 1622 (imidazole C=O), 1600 - 1511 (C=C Ar), 1232 (C-N) 855 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.87 ( s, C=CH- ), 8.43 (s, NH2), 6.91 – 8.1 (m, Aromatic protons)
2.5.2. (4b). 3-Amino-5-(4-Nitrobenzylidene)-2-(Pyridin-3- yl)-3,5-Dihydro-4h-Imidazol-4-One
- (yield 46 %), (m.p, C°,200-201), IR. (KBr) (ν, Cm-1) 3371-3284 (NH2), 3099 (C-H ar), 3222 (C-H olifen), 1626 (imidazole C=O), 1606 - 1516 (C=C Ar), 1213 (C-N) 848 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.75 ( s, C=CH- ), 8.51 (s, NH2), 721 – 8.00 (m, Aromatic protons)
2.6. Synthesis of 5-(Arylidene)-3-[(Arylidene) Amino]-2-(Pyridin-3-yl)-3,5-Dihydro-4H-Imidazol-4-One (5a – D)
- The corresponding aryl aldehyde (0.01 mol) was added to a stirred solution of compound (4a – b) (0.01 mol) in absolute ethanol (20ml) and the mixture was refluxed for 2h. .After cooling, the mixture was filtered and the solid recrystallized from ethanol to afford the desired compound.
2.6.1. (5a). 5-(4-Bromobenzylidene)-3-[(4-Nitrobenzylidene) Amino]-2-(Pyridin-3-yl)-3,5-Dihydro-4h-Imidazol-4-One
- (yield 83 %), (m.p, C°, 242-245), IR. (KBr) (ν, Cm-1) 3028 (C-H Ar), 3284 (C-H olifen), 1628 (imidazole C=O),1654 (C=N, Schiff `s base )1604 - 1519 (C=C Ar), 1267 (C-N) 850 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.65 ( s, C=CH- ), 8.91 (s, CH=N), 6.54 – 8.12 (m, Aromatic protons).
2.6.2. (5b). 5-(4-Bromobenzlidene)-3- [(4-Bromobenzylidene) Amino]-2-(Pyridin-3-yl)-3, 5-Dihydro-4H-Imidazol-4-One
- (yield 87 %), (m.p, C°, 192-194), IR. (KBr) (ν, Cm-1) 3062 (C-H Ar), 3190 (C-H olifen), 1631 (imidazole C=O),1663 (C=N, Schiff `s base )1600 - 1523 (C=C Ar), 1278 (C-N) 841 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.71 ( s, C=CH- ), 8.95 (s, CH=N), 6.43 – 8.22 (m, Aromatic protons).
2.6.3. (5c). 5-(4-Nitrobenzylidene)-3-[(4-Bromobenzylidene) Amino]-2-(Pyridin-3-yl)-3,5-Dihydro-4H-Imidazol-4-One
- (yield 74 %), (m.p, C°, 143-146), IR. (KBr) (ν, Cm-1) 3080 (C-H Ar), 3122 (C-H olifen), 1632 (imidazole C=O),1645 (C=N, Schiff `s base )1611 - 1500 (C=C Ar), 1230 (C-N) 813 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.72 ( s, C=CH- ), 8.98 (s, CH=N), 6.32 – 7.98 (m, Aromatic protons).
2.6.4. (5d). 5-(4-Nitrobenzylidene)-3-[4-Nitrobenzylidene) Amino]-2-(Pyridin-3-yl)-3,5-Dihydro-4H-Imidazol-4-One
- (yield 79 %), (m.p, C°, 145-147), IR. (KBr) (ν, Cm-1) 3069 (C-H Ar), 3200 (C-H olifen), 1636 (imidazole C=O),1666 (C=N, Schiff `s base )1601 - 1514 (C=C Ar), 1228 (C-N) 858 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.45 ( s, C=CH- ), 8.87 (s, CH=N), 6.51 – 8.17 (m, Aromatic protons).
2.7. Synthesis of N-[Chloro (Aryl) Methyl]-N-[Arylidene-5-Oxo-2-(Pyridin-3-yl )-4, 5-Dihydro-1H-Imidazol-1-yl]Benzamide (6a - b )
- To a stirring solution of compound (5a - b ) (0.003 mol) in dry benzene (15 ml),benzoyl chloride (0.003 mole, 0.35 g) in benzene (10 ml) was added drop wise, then the mixture was refluxed for (4 hrs.) with stirring. After cooling, the precipitate crystals was filtered and recrystallized from ethanol
2.7.1. (6a). N-[chloro (4-Bromophenyl) Methyl]- N-[4-Nitrobenzylidene-5-oxo-2-(Pyridin-3-yl )-4, 5-Dihydro-1H-Imidazol-1-yl]Benzamide
- (yield 62 %), (m.p, C°)(170-173), IR. (KBr) (ν, Cm-1) 3036 (C-H Ar), 3123 (C-H olifen), 1642 (imidazole C=O),1651 (C=O, amide )1588 - 1481 (C=C Ar), 1205 (C-N) 858 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.52 ( s, C=CH- ), 4.76 (s, N-CH-Cl), 6.79 – 8.42 (m, Aromatic protons).
2.7.2. (6b). N-[chloro (4-Nitrophenyl) Methyl]-N-[4-Bromobenzylidene-5-oxo-2-(Pyridin-3-yl )-4,5-Dihydro-1H-Imidazol-1-yl]Benzamide
- (yield 71 %), (m.p, C°)(190-192), IR. (KBr) (ν, Cm-1) 3044 (C-H Ar), 3149 (C-H olifen.), 1634 (imidazole C=O),1664 (C=O, amide )1603 - 1524 (C=C Ar), 1267 (C-N) 879 (para substitution); 1HNMR (DMSO-d6) Ϩ (ppm) 8.75 ( s, C=CH- ), 5.21 (s, N-CH-Cl), 715 – 8.26 (m, Aromatic protons).
2.8. Synthesis of Aryl{[4-Arylidene-5-oxo-2-(Pyridin-3- yl)-4,5-Dihydro-1H-Imidazol-1-yl](Benzoyl)Amino}Methyl Carbamimidothioate(7a - b)
- A mixture of compounds (6a - b) (0.005 mole) thiourea (0.005mole,0.44 gm) and anhydrous sodium carbonate (0.005 mole) in absolute ethanol (20 ml) was refluxed for 5 hrs. with stirring and the precipitated crystals was filtered and recrystallized from appropriate solvent.
2.8.1. (7a). 4-Bromophenyl{[4-(4-Nitrobenzylidene-5-oxo- 2-(Pyridin-3-yl)-4,5-Dihydro-1H-Imidazol-1-yl] (Benzoyl)Amino}Methyl Carbamimidothioate
- (yield 58 %), (m.p, C°)(134-136), IR. (KBr) (ν, Cm-1) 3385-3277 (NH2), 3182 (NH) 3049 (C-H Ar), 3112 (C-H olefene.), 1627 (imidazole C=O),1666 (C=O, amide )1608 - 1527 (C=C Ar), 1297 (C-N) 858 (para substitution); 1HNMR (DMSO-d6) Ϩ (ppm) 8.65 (s, NH2), 8.43 ( s, C=CH- ), 4.21 (s, N-CH-S), 6.11 – 7.86 (m, Aromatic proton).
2.8.2. (7b). 4-Nitrophenyl-{[4-(4-Bromobenzylidene-5-oxo- 2-(Pyridin-3-yl)-4,5-Dihydro-1H-Imidazol-1-yl] (Benzoyl)Amino}Methyl Carbamimidothioate
- (yield 65 %), (m.p, C°, 165-167), IR. (KBr) (ν, Cm-1) 3362-3269 (NH2), 3169 (NH) 3060 (C-H Ar), 3102 (C-H olefene.), 1628 (imidazole C=O),1643 (C=O, amide )1589- 1473 (C=C Ar), 1294 (C-N) 823 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.75 (s, NH2), 8.52 ( s, C=CH- ), 3.86 (s, N-CH-S), 6.46 – 8.12 (m, Aromatic proton)).
2.9. Synthesis of N-{1-[(4,6-Dioxo-1,4,5,6-Tetrahydropyrimidin-2-yl) Sulfanyl]Benzyl}-N-[Arylidene-5-oxo-2-(Pyridin-3-yl)-4,5-Dihydro-1H-Imidazol-1-yl-Benzamide (8a - b)
- A mixture of compounds (7a - b) (0.005mole) diethylmalonate (0.005mole, 0.8 gm.) and anhydrous sodium carbonate (0.005 mole) in dry benzene (20 ml) was refluxed for 7 hrs. with stirring , the product was collected as oily, it is purified by column chromatography with silica gel and a mixture of EtOH : benzene as eluent.
2.9.1. (8a). N-{1-[(4,6-Dioxo-1,4,5,6-Tetrahydropyrimidin- 2-yl) Sulfanyl]Benzyl}-N-[4-Bromobenzylidene-5- oxo-2-(Pyridin-3-yl)-4,5-Dihydro-1H-Imidazol-1-yl-Benzamide
- (yield 55 %), (m.p, C°, 297-299),IR. (KBr) (ν, Cm-1) 3248 (NH) 3044 (C-H Ar), 3176 (C-H olefene.), 2978-2845 (C-H Aliph.), 1701 (Pyrimidine C=O), 1639 (imidazole C=O), 1665 (C=O, amide )1600-1501 (C=C Ar), 1241 (C-N) 825 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.57 (s, NH), 8.13 ( s, C=CH), 3.56 (s, N-CH-S), 4.42 (s,NH-CH2-NH) 6.11 – 7.94 (m, Aromatic proton).
2.9.2. (8b). N-{1-[(4,6-Dioxo-1,4,5,6-Tetrahydropyrimidin- 2-yl) Sulfanyl]Benzyl}-N-[4-Nitrobenzylidene-5-oxo- 2-(Pyridin-3-yl)-4,5-Dihydro-1H-Imidazol-1-yl- Benzamide
- (yield 63 %), (m.p, C°, 231-233),IR. (KBr) (ν, Cm-1) 3202 (NH) 384 (C-H Ar), 3154 (C-H olefene.), 2990-2849 (C-H Aliph.), 1704 (Pyrimidine C=O), 1631 (imidazole C=O), 1665 (C=O, amide )1608-1511 (C=C Ar), 1291 (C-N) 832 (para substitution); 1HNMR (DMSO-d6) ς (ppm) 8.69 (s, NH), 8.21 ( s, C=CH), 3.71 (s, N-CH-S), 4.40 (s,NH-CH2-NH), 6.15–8.02 (m, Aromatic proton).
2.10. Synthesis of Ethyl {[(4Z)-4-(Arylidene)-5-oxo-2-(Pyridin-3-yl)-4,5- Dihydro-1H-Imidazol-1-yl]Amino}Acetate 9a-c
- The corresponding compound 7 (0.01mole) was refluxed with an equivalent amount of sodium in absolute ethanol for 2h. Then, ethyl bromoacetate (1.81 g, 0.01mole) was added and refluxed for an additional 5h. Having evaporated it under reduced pressure, a solid appeared this was recrystallized from ethanol to afford the desired compound.
2.10.1. (9a). ethyl {[(4Z)-4-(4-bromobenzylidene)-5-oxo-2- (pyridin-3-yl)-4,5-dihydro-1H-imidazol-1-yl]amino}acetate
- (yield 76 %), (m.p, C°, 222-224), IR. (KBr)(ν,Cm-1) 3163 (NH), 3080 (C-Har), 2993-2885 (C-H alph.), 1741 (ester C=O), 1692 (imidazole C=O), 1265 (C-O); 1HNMR (DMSO-d6) ς (ppm) 1.47-176 (t, CH3-CH2-), 2.25-2.69 (t, N-CH2), 3.22 (s, C=CH), 3.11-3.29 (q, CH2-CH3), 3.72-3.89, 5.25 (s, N-CH2-CO), 6.42-7.12 (d, 2H, ArH), 7.41-7.92 (d, 2H, ArH), 8.53 (s, 1H, imidazole), 10.62 (s, NH).
2.10.2. (9b). ethyl {[(4Z)-4-(4-nitrobenzylidene)-5-oxo-2- (pyridin-3-yl)-4,5-dihydro-1H-imidazol-1-yl]amino} acetate
- (yield 85 %), (m.p, C°)(176-178), IR. (KBr)(ν,Cm-1) 3154 (NH), 3066 (C-Har), 2946-2858 (C-H alph.), 1723 (ester C=O), 1683 (imidazole C=O), 1612 (C=C alkene) 1290 (C-O); 1HNMR (DMSO-d6) ς (ppm) 1.12-156 (t, CH3-CH2-), 2.26-2.35 (t, N-CH2), 3.18 (s, C=CH), 3.41-3.69 (q, CH2-CH3), 5.14 (s, N-CH2-CO), 6.69-7.07 (d, 2H, ArH), 7.47-7.81 (d, 2H,
2.11. Synthesis of N'-[(amino-sulfanylidyne) methyl]-2-{[(4Z)-4-(arylidene)-5-oxo-2-(pyridin-3-yl)-4,5-dihydro-1H-imidazol-1-yl] amino} acetohydrazide 10a-b
- A mixture of compound 9a - b (0.01, mole) and thiosemicarbazide, ( 0.91 g, 0.01 mole) in ethanol (25 ml) was refluxed for 8h.The solution on cooling a solid appeared. This was recrystallized from ethanol to afford the desired compound.
2.11.1. (10a). N'-[(amino-sulfanylidyne)methyl]-2-{[(4Z)-4- (4-bromo benzylidene)-5-oxo-2-(pyridin-3-yl)-4, 5-dihydro-1H-imidazol-1-yl] amino}acetohydrazide
- (yield 61 %), (m.p, C°)(212-214), IR. (KBr)(ν,Cm-1) 3410-3375 (NH2), 3247 (thiosemicarbazide NH), 3199 (NH), 3056 (C-Har), 2971-2850 (C-H alph.), 1688 (imidazole C=O), 1641 (amide C=O), 1243 (C=S); 1HNMR (DMSO-d6) ς (ppm) 2.16-2.52 (t, N-CH2), 3.18 (s, C=CH), 4.83 (s, N-CH2-CO), 6.55 (s, NH2), 6.78-6.91 (d, 2H, ArH), 7.34-7.85 (d, 2H, ArH), 8.86 (s, 1H, imidazole), 10.15 (s, NH), 10.84 (s, N-NH-CS), 11.42 (s, CO-NH-N).
2.11.2. (10b). N'-[(amino-sulfanylidyne)methyl]-2- {[(4Z)-4-(4-nitro benzylidene)-5-oxo-2-(pyridin-3- yl)-4,5-dihydro-1H-imidazol-1-yl] amino} acetohydrazide
- (yield 59 %), (m.p, C°,254-256), IR. (KBr)(ν,Cm-1) 3421-3397 (NH2), 3286 (thiosemicarbazide NH), 3226 (NH), 3060 (C-Har), 2978-2899 (C-H alph.), 1704 (imidazole C=O), 1664 (amide C=O), 1243 (C=S); 1HNMR (DMSO-d6) ς (ppm) 2.32-2.77 (t, N-CH2), 3.21 (s, C=CH), 4.45 (s, N-CH2-CO), 5.93 (s, NH2), 6.51-6.78 (d, 2H, ArH), 7.43-7.92 (d, 2H, ArH), 8.43 (s, 1H, imidazole), 10.28 (s, NH), 10.67 (s, N-NH-CS), 11.19 (s, CO-NH-N).
2.12. Synthesis of (5Z)-3-{[(5-amino-1,3,4-thiadiazol-2- yl)methyl]amino}-5-(arylidene)-2-(pyridin-3-yl)-3, 5-dihydro-4H-imidazol-4-one 11a-b
- Corresponding compounds 10a - b (0.01mole) was dissolved in cold conc. Sulfuric acid (10 ml) and stirred at room temperature for 24h. Then, reaction mixture was poured into crushed ice and diluted with water, the precipitate was filtered, washed with water and recrystallized from ethanol to afford the desired compound.
2.12.1. (11a0. (5Z)-3-{[(5-amino-1,3,4-thiadiazol-2-yl) methyl]amino}-5-(4-bromobenzylidene)-2-(pyridin-3-yl)-3,5-dihydro-4H-imidazol-4-one
- (yield 63 %), (m.p, C°,174-176), IR. (KBr)(ν,Cm-1) 3432-3386 (NH2), 3210 (NH), 3042 (C-Har), 2986-2879 (C-H alph.), 1651 (imidazole C=O); 1HNMR (DMSO-d6) ς (ppm) : 3.62 (s, C=CH), 4.85 (s, N-CH2-Thiadiazole), 6.11 (s, NH2), 6.51-6.82 (d, 2H, ArH), 7.45-7.97 (d, 2H, ArH), 8.23(s, 1H,imidazole), 10.31 (s, NH).
2.12.2. (11b). (5Z)-3-{[(5-amino-1,3,4-thiadiazol-2-yl) methyl]amino}-5-(4-bromobenzylidene)-2-(pyridin-3-yl)-3,5-dihydro-4H-imidazol-4-one
- (yield 71 %), (m.p, C°,281-283), IR. (KBr)(ν,Cm-1) 3427-3388 (NH2), 3215 (NH), 3049 (C-Har), 2991-2890 (C-H alph.), 1701 (imidazole C=O); 1HNMR (DMSO-d6) ς (ppm) : 3.29 (s, C=CH), 4.58 (s, N-CH2-Thiadiazole), 6.21 (s, NH2), 6.76-6.81 (d, 2H, ArH), 7.46-7.78 (d, 2H, ArH), 8.25(s, 1H,imidazole), 10.66 (s, NH).
2.13. Synthesis of 2-{[(4Z)-4-(arylidene)-5-oxo-2- (pyridin-3-yl)-4,5-dihydro-1H-imidazol-1-yl]amino} acetohydrazide 12a-b
- A mixture of compound 9a - b (0.01, mole) and hydrazine hydrate(99% , 0.32 g, 0.01 mole) in ethanol (25 ml) was refluxed for 8h.The solution on cooling a solid appeared. This was recrystallized from ethanol to afford the desired compound.
2.13.1. (12a). 2-{[(4Z)-4-(4-bromobenzylidene)-5-oxo-2- (pyridin-3-yl)-4,5-dihydro-1H-imidazol-1-yl]amino} acetohydrazide
- (yield 74 %), (m.p, C°,162-164), IR. (KBr)(ν,Cm-1) 3400-3374 (NH2), 3230 (NH), 3071 (C-Har), 2948-2826 (C-H alph.), 1699 (imidazole C=O),1670 (amide C=O), 1232 (C-N); 1HNMR (DMSO-d6) ς (ppm) 2.36-2.67 (t, N-CH2), 3.43(s, C=CH), 4.28 (s, N-CH2-CO), 6.22 (s, NH2), 6.71-7.18 (d, 2H, ArH), 7.42-7.893 (d, 2H, ArH), 8.52 (s, 1H, imidazole), 10.74 (s, NH), 11.91 (s, CO-NH-N).
2.13.2. (12b). 2-{[(4Z)-4-(4-nitrobenzylidene)-5-oxo-2- (pyridin-3-yl)-4,5-dihydro-1H-imidazol-1-yl]amino} acetohydrazide
- (yield 62 %), (m.p, C°,186-188), IR. (KBr)(ν,Cm-1) 3431-3383 (NH2), 3210 (NH), 3088 (C-Har), 2992-2879 (C-H alph.), 1690 (imidazole C=O),1657 (amide C=O), 1242 (C-N); 1HNMR (DMSO-d6) ς (ppm) 2.56-2.89 (t, N-CH2), 3.40(s, C=CH), 4.58 (s, N-CH2-CO), 6.52 (s, NH2), 6.70-6.82 (d, 2H, ArH), 7.44-7.89 (d, 2H, ArH), 8.47 (s, 1H, imidazole), 10.57 (s, NH), 11.02 (s, CO-NH-N).
2.14. Synthesis of (5Z)-5-(arylidene)-2-(pyridin-3-yl)-3- {[(5-sulfanyl-1,3,4-oxadiazol-2-yl)methyl]amino}-3, 5-dihydro-4H-imidazol-4-one 13a-b
- Corresponding compound 12a - b (0.01mole) and CS2 (0.6 ml, 0.01 mole) were added to a solution of KOH (0.56 g, 0.01 mole) in (30ml) ethanol. The reaction mixture was refluxed for 3h. After evaporation it in reduced pressure to dryness, a solid obtained. This was dissolved in 200 ml H2O and acidified with conc. HCl. The precipitate was filtered off, washed with water and recrystallized from ethanol to afford the desired compound.
2.14.1. (13a0. (5Z)-5-(4-bromobenzylidene)-2-(pyridin-3- yl)-3-{[(5-sulfanyl-1,3,4-oxadiazol-2-yl)methyl] amino}-3,5-dihydro-4H-imidazol-4-one
- (yield 44 %), (m.p, C°,193-195), IR. (KBr)(ν,Cm-1) 3198 (NH), 3075 (C-Har), 2982-2893 (C-H alph.), 2466 (SH), 1685 (imidazole C=O), 1291 (C=S); 1HNMR (DMSO-d6) ς (ppm) 2.41-2.64 (t, N-CH2), 3.34 (s, C=CH), 4.45 (s, N-CH2-oxadiazole), 6.71-6.94 (d, 2H, ArH), 7.59-7.93 (d, 2H, ArH), 8.74 (s, 1H, imidazole), 10.61 (s, NH), 12.04 (s, NH + SH of oxadiazole).
2.14.2. (13b). (5Z)-5-(4-nitrobenzylidene)-2-(pyridin-3-yl)- 3-{[(5-sulfanyl-1,3,4-oxadiazol-2-yl) methyl] amino}-3, 5-dihydro-4H-imidazol-4-one
- (yield 60 %), (m.p, C°)(255-257),IR. (KBr)(ν,Cm-1) 3206 (NH), 3053 (C-Har), 2991-2890 (C-H alph.), 2465 (SH), 1668 (imidazole C=O), 1272 (C=S); 1HNMR (DMSO-d6) ς (ppm) 2.33-3.02 (t, N-CH2), 3.61 (s, C=CH), 4.42 (s, N-CH2-oxadiazole), 6.54-6.69 (d, 2H, ArH), 7.41-7.87 (d, 2H, ArH), 8.27 (s, 1H, imidazole), 10.28 (s, NH), 12.94 (s, NH + SH of oxadiazole).
2.15. Synthesis of (5Z)-3-{[(4-amino-5-sulfanyl-4H-1,2, 4-triazol-3-yl)methyl]amino}-5-(arylidene)-2- (pyridin- 3-yl) -3,5-dihydro-4H-imidazol-4-one 14a-b
- Corresponding compound 12a – b (0.01mole) and CS2 (0.6 ml, 0.01 mole) in (20 ml) ethanol. The reaction mixture was stirred for 12h. Then, diethyl ether (18 ml) was added to it. The precipitated solid thus obtained was filtered, washed with cold diethyl ether, without isolation and purification dissolved in water (10 ml), hydrazine hydrate (99%) (0.34g, 0.01 mole) was added. The reaction mixture was refluxed for 1h. cooled, diluted with water and acidified with acetic acid. The precipitate was filtered off, washed with water and recrystallized from ethanol to afford the desired compound.
2.15.1. (14a).(5Z)-3-{[(4-amino-5-sulfanyl-4H-1,2, 4-triazol -3-yl) methyl]amino }-5-(4- bromobenzylidene) -2-(pyridin-3-yl)-3, 5-dihydro-4H-imidazol-4-one
- ( yield 66 %), (m.p, C°,204-206), IR. (KBr)(ν,Cm-1) 3412-3352 (NH2), 3270 (NH), 3066 (C-Har), 2982-2887 (C-H alph.), 2440 (SH), 1692 (imidazole C=O), 1255 (C=S); 1HNMR (DMSO-d6) ς (ppm) 2.43-2.69 (t, N-CH2), 3.40 (s, C=CH), 4.91 (s, N-CH2-Triazole), 6.52 (s, NH2), 6.72-6.98 (d, 2H, ArH), 7.49-7.81 (d, 2H, ArH), 10.69 (s, NH), 13.01 (s, NH + SH
2.15.2. (14b). (5Z)-3-{[(4-amino-5-sulfanyl-4H-1,2,4- triazol-3-yl)methyl]amino }-5-(4- mitrobenzylidene) -2- (pyridin-3-yl)-3, 5-dihydro-4H-imidazol-4-one.
- (yield 57 %), (m.p, C°,290-292), IR. (KBr)(ν,Cm-1) 3439-3312 (NH2), 3178 (NH), 3059 (C-Har), 2985-2870 (C-H alph.), 2427 (SH), 1704 (imidazole C=O), 1219 (C=S); 1HNMR (DMSO-d6) ς (ppm) 2.51-2.78 (t, N-CH2), 3.35 (s, C=CH), 4.62 (s, N-CH2-Triazole), 6.13 (s, NH2), 6.71-6.95 (d, 2H, ArH), 7.51-7.87 (d, 2H, ArH), 8.82 (s, 1H, imidazole), 10.58 (s, NH), 13.10 (s, NH + SH of triazole).
3. Results and discussion
- Schemes (1) were summarized the synthesis of different derivatives of nicotinic acid.Nicotinoyl chloride (1) was synthesized by treatment of nicotinic acid with thionyl chloride. The reaction is followed by the appearance of (υC=O) absorption bands at (1799) cm-1 in their spectra.[(Pyridyl-3-yl-carbonyl amino]acetic acid (2)[13] have been synthesized by the reaction of compound (1) with glycine in the presence of sodium hydroxide through nucleophilic displacement mechanism (SN2).The reaction is followed by decreasing of absorption band for (υC=O ) at 1741 cm-1 and appearance of new absorption band at 3180 cm-1 due to (υNH). In the 1H NMR spectra, the proton signals due to (CO-CH2-NH) was recorded at 4.42 ppm integrating for two protons. The treatment of compound (2) with aryl aldehyde in presence of acetic acid and acetic anhydride led to the formation of4-(arylidene)-2-(pyridin-3-yl)-1,3-oxazol-5(4H)-one(3a- b).[14] Compounds (3a – b) have been identified by IR spectrum which it showed the appearance of characteristic absorption bands near 1755-1770 cm-1 which belonged to the oxazol-5(4H)-one carbonyl group (oxazole, υC=O), and at 3123-3220 cm-1 due to (olifinic υCH), 1H NMR spectra showed signals at 8.82-8.90 ppm due to (CH, olifenic) and at 6.21-8.10 ppm which belonged to aromatic protons. Refluxing compounds (3a – b) with hydrazine hydrate (99%) for 20 hrs offered good yields of the corresponding derivatives (4a - b). The IR spectra of compounds (4a - b) displaced peaks at 1622-1626 cm-1, 3227-3424 cm-1 for (imidazole, υC=O) and (υNH2) functions respectively. 1H NMR spectra showed signals at 8.43-851 ppm due to (NH2), at 8.75-8.87 ppm for (CH, olifenic) and at 6.91 – 8.1ppm which belonged to aromatic protons. The formation of Schiff's bases (5a - d) was confirmed by the absence of absorption bands near 3227-3424 cm-1 for (υNH2) and presence of absorption bands at 1645-1666 cm-1 due to (υC=N) stretching. 1HNMR spectra of compound (5a – d) show the new signals observed at 8.87-8.98 ppm integrating for (CH=N) and at 6.32-8.22 ppm integrating for protons assigned to aryl groups.When the Schiff's bases (5a - b) were treated with benzoyl chloride (scheme 2) in boiling benzene, derivative (6a - b) was obtained in good yield. The IR spectrum indicated the presence of a (υCON) function (1651-1664) cm-1 and at 1642-1634 cm-1due to(imidazole (υC=O) in addition to (olifinic υCH) at 3123-3149 cm-1. 1H NMR spectra showed signals at 8.52-8.75 ppm due to (CH, olifenic), at 6.79-8.42 ppm which belonged to aromatic protons and at 4.76-5.21 for ( N-CH-Cl). On the other hand, when the derivatives (6a - b) was treated with thiourea, derivatives (7a - b) was obtained in good yield. The IR spectra indicated the presence of a doublet absorption bands near (3385-3269) cm-1 for (υNH2). . 1H NMR spectra showed signals at 8.65-8.75 ppm due to (NH2), at 6.11-8.12 ppm which belonged to aromatic protons and at 3.86-4.21 for ( N-CH-S).
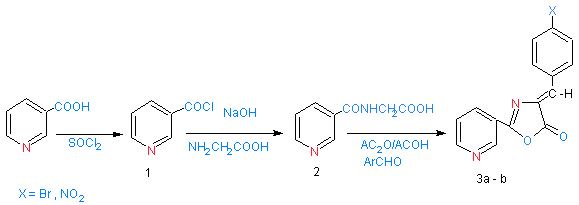 | Scheme 1. shows synthesized of compounds 3a- b |
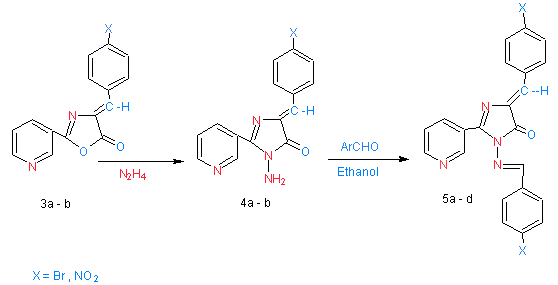 | Scheme 2. shows synthesized of compounds 5a- b |
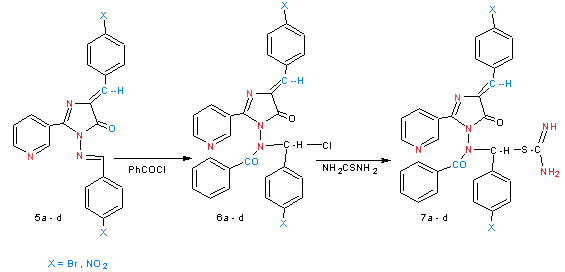 | Scheme 3. shows synthesized of compounds 6a - d and 7a - d |
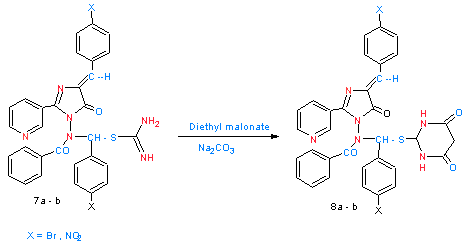 | Scheme 4. shows synthesized of compounds 8a- b |
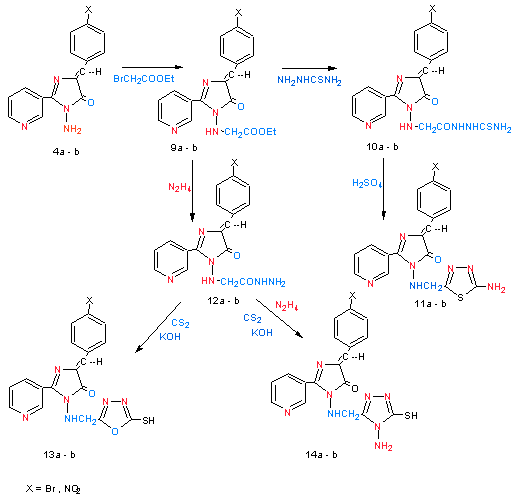 | Scheme 5. shows synthesized of compounds (9a – b) – (14a – b) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML