| [1] | Soderberg, B. C. G. Coord. Chem. Rev. (2003), 241, 147. |
| [2] | (a) Fürstner, A., Bogdanovic, B. Angew. Chem. Int. Ed. Engl.(1996),35, 2442. (b) Gansauer, A.; Bluhm, H. Chem. Rev. (2000), 100, 2771. (c) Armbruster, J.; Grabowski, S.; Ruch, T.; Prinzbach, H. Angew. Chem. Int. Ed. Engl. (1998), 37, 2242. (d) McMurry, J. E. Chem. Rev. (1989), 89, 1513. (e) Kahn, B. E ; Rieke, R. D. Chem. Rev. (1988), 88, 733. (f) Ephritikhine, M.; Villiers, C. in Modern Carbonyl Olefination,Takeda, T. Ed., Wiley-VCH, Weinheim, (2004), 223. (g) Fürstner, A., in Transition Metals for Organic Synthesis (2nd Ed.), Beller, M.; Bolm, C. Eds., Wiley-VCH, Weinheim, (2004), 1, 449. (h) Dushin, R. G. in Comprehensive Organometallic Chemistry II, Hegedus, L. S. Ed., ergamon, Oxford,(1996 ), 12, 1071. (i) Robertson, G. M. In Comprehensive Organic Synthesis I, Trost, B.M. Ed. Pergamon, New York, (1991), 3, 563. |
| [3] | Gao, Y.; Yoshida, Y.; Sato, F. Tetrahedron Lett. (1996), 37, 7787–7790. |
| [4] | Gao, Y.; Yoshida, Y.; Sato, F. Synlett (1997), 1353–1354. |
| [5] | For selected reviews, see: (a) Bellina, F.; Rossi, R. Tetrahedron (2006), 62, 7213–7256. (b) Balme, G. Angew. Chem., Int. Ed. (2004), 43, 6238–6241. (c) Fuerstner, A. Angew. Chem., Int. Ed. (2003), 42, 3582–3603. (d) Hoffmann, H.; Lindel, T. Synthesis (2003), 1753–1783. (e) Novak, P.; Mueller, K.; Santhanam, K. S. V.; Haas, O. Chem. Rev. (1997), 97, 207–281. |
| [6] | (a) McMurry, J. E.; Chem. Rev. (1989), 1513-1524. (b) Lect, ka, T. in Active metals Preparations., Characterization, Applications (Furstner, Ed.) VCH, Weinheim. (1995), 85-131. |
| [7] | (a) Furstner, A; Jumbam, D. N.; Tetrahedron (1992), 48, 5991-6010. (b) Furstner, A.; Jumbam, D. N.; Wiedmann, H.; Tetrahedron Lett. (1991), 6695-6696. (c) Furstner, A.; Jumbam, D. N; J. Chem. Soc. Chem. Commun. (1993), 211-212. (d) Furstner, A; Jumbam, D. N.; Seidel, J.; Chem. Ber. (1994), 1125-1130. (e) Furstner, A.; Weintritt, H.; Hupperts, A.; J. Org. Chem. (1995), 60, 6637-6641. |
| [8] | (a) Furstner, A.; Hupperts, A.; Ptock, A.; Jansen, E.; J. Org. Chem. (1994), 59, 5215-5229. (b) Furstner, A.; Ptock, A.; Weintritt, H.; Goddard, R.; Kruger, C.; Angew. Chem. Int. (1995), 34, 725-728. (c) Furstner, A.; Ernst, A.; Tetrahedon (1995), 51, 773-786. |
| [9] | Furstner, A; Hupperts, A.; J. Am. Chem. Soc. (1995), 117, 4468-4475 . |
| [10] | Ackermann, L.; Organometallics (2003), 22, 4367-4368. |
| [11] | Ackermann, L.; Born, R.; Tetrahedron Letter (2004), 45, 9541–9544. |
| [12] | Abbiati, G.; Casoni, A.; Canevari, V.; Nava, D.; Rossi, E.; Org. Lett. (2006), 8, 4839-4842. |
| [13] | Ackermann, L.; Sandmann, R; Villar, A; Kaspar, T. L.; Tetrahedron (2008), 64, 769-777. |
| [14] | Pohlki, F.; Doye, S.; Chem. Soc. Rev. (2003), 32, 104-114. |
| [15] | For a first titanium-catalyzed intermolecular hydroamination of an alkyne, see: (a) Hill, J. E.; Profilet, R. D.; Fanwick, P. E.; Rothwell, I. P. Angew. Chem. Int. Ed. Engl. (1990), 29, 664-665. (b) McGrane, P. L.; Jensen, M.; Livinghouse, T.; J. Am. Chem. Soc. (1992), 114, 5459-5460. (c) McGrane, P. L.; Livinghouse, T.; J. Org. Chem.(1992), 57, 1323- 1324. (d) Polse, J. L.; Andersen, R. A.; Bergman, R. G.; J. Am. Chem. Soc. (1998), 120, 13405-13414. |
| [16] | Pohlki, F.; Doye, S.; Chem. Soc. Rev. (2003), 32, 104-114. |
| [17] | (a) Walsh, P. J.; Hollander, F. J.; Bergman, R. G.; J. Am. Chem. Soc. (1988), 110, 8729-8731. (b) Walsh, P. J.; Baranger, A. M.; Bergman, R. G.; J. Am. Chem. Soc. (1992), 114, 1708-1719. (c) Duncan, A. P.; Bergman, R. G.; Chem. Rec. (2002), 2, 431-445. |
| [18] | (a) Buil, M. L.; Esteruelas, M. A.; Lopez,A. M.; Mateo, A. C.; Onate, E.; Organometallics (2007), 26, 554-565. (b) Swartz, D. L.; Odom, A. L.; Organometallics (2006), 25, 6125-6133. (c) Smolensky, E.; Kapon, M.; Eisen, M. S.; Organometallics (2005), 24, 5495-5498. (d) Tillack, A.; Khedkar, V.; Jiao, H.; Beller, M.; Eur. J. Org. Chem. (2005), 5001-5012. (e) Hoover, J. M.; Petersen, J. R.; Pikul, J. H.; Johnson, A. R.; Organometallics (2004), 23, 4614-4620. (f) Heutling, A.; Pohlki, F.; Doye, S.; Chem. Eur. J. (2004), 10, 3059-3071. (g) Zhang, Z.; Schafer, L. L.; Org. Lett. (2003), 5, 4733-4736. (h) Lorber, C.; Choukroun, R.; Vendier, L. Organometallics (2004), 23, 1845-1850. (i) Shi, Y.; Ciszewski, J. T.; Odom, A. L.; Organometallics (2001), 20, 3968-3969. |
| [19] | (a) Odom, A. L.; Dalton Trans. (2005), 225-233. (b) Bytschkov, I.; Doye, S.; Eur. J. Org. Chem. (2003), 935-946. |
| [20] | (a) Ackermann, L.; Organometallics (2003), 22, 4367-4368. (c)Ackermann, L.; Kaspar, L. T.; Gschrei, C.; J. Chem. Commun. (2004), 2824- 2825. |
| [21] | (a) Beller, M.; Seayad, J.; Tillack, A.; Jiao, H. Angew. Chem., Int. Ed.), (2004), 43, 3368-3398. (b) Alonso, F.; Beletskaya, I. P.; Yus, M. Chem. Rev. (2004), 104, 3079-3159. |
| [22] | Ackermann, L. Kaspar,T. L. J. Org. Chem. (2007), 72, 6149-6153. |
| [23] | Shi, D. Q.; Shi, C. L.; Wang, X. S.; Zhuang, Q. Y.; Tu, S. J.; Hu, H. W.; Synlett (2004), 2239–2241. |
| [24] | Shi, D. Q; Shi, C. L.; Dou, G.; Zingy, Li.; Synthesis (2007), 20, 3117- 3124. |
| [25] | Rao, H. S. P.; Jothilingam, S.; Scheeren, H. W. Tetrahedron ( 2004), 60, 1625–1630 |
| [26] | Shi, D. Q.; Shi, C. L.; Wang, X. S.; Zhuang, Q. Y.; Tu, S. J.; Hu, H. W. Synlett. (2004), 2239–2241. |
| [27] | Shi, D. Q.; Dou, G. L.; Shi, C. L.; Li, Z. Y.; Ji, S. J. Synthesis ( 2007), 20, 3117–3124. |
| [28] | Dou, G.; Shi, C.; Shi, D.; J. Combinatorial Chem. (2008), 10, 811- 813. |
| [29] | Ackermann, L.; Sandmann, R.; Kaspar, L. T; Org. Lettt (2009), 11, 2031. |
| [30] | Duan, F. X.; Bin Zhang, B. Z. Z. J; Fu, G. Ziy, Beijing J. Org. Chem. (2007), 72, 10283-10286. |
| [31] | Abbiati, G.; Casoni, A.; Canevari, V.; Nava, D.; Rossi, E. Org. Lett. (2006), 21, 4839-4842 |
| [32] | Shi, D. Q.; Feng, S.; Rong, Dou, G. L.; Synth. Commun, (2010), 40, 2302–2310. |
| [33] | Rajanbabu, T. V.; Nugent, W. A. J. Am. Chem. Soc. (1994), 116, 986. |
| [34] | (a) Gansauer, A.; Bauer, D. J. Org. Chem. (1998), 63, 2070. (b) Yamamoto, Y.; Hattori, R.; Miwa, T.; Nakagai, Y.; Kubota, T.; Yamamoto, C.; Okamoto, Y.; Itoh, K. J. Org. Chem. (2001), 66, 3865. |
| [35] | Zhou, L.; Hirao, T.; J. Org. Chem. (2003), 68, 1633-1635. |
| [36] | (a) Gansauer, A.; Bauer, D. J. Org. Chem. (1998), 63, 2070. |
| [37] | Jana, S.; Roy, S. C.; Tetrahedron Lett. (2006), 47, 5949–5951. |
| [38] | Wipf, P.; Maciejewski, P. J.; Org. Lett. (2008), 10, 4383-4386. |
| [39] | Maciejewski, J. P.; Wipf, P. ARKIVOC (2011), 6, 92-111. |
| [40] | Okazoe, T.; Takai, K.; Oshima, K.; Utimoto, K. J. Org. Chem. (1987), 52, 4410–4412. |
| [41] | Petasis, N. A.; Bzowej, E. I. J. Am. Chem. Soc. (1990), 112, 6392–6394. |
| [42] | Bennasar, M. L.; Roca, M.; Manuel Monerris, M.; Dıaz, D. G. Tetrahedron Lett. (2005), 46, 4035–4038. |
| [43] | Macleod, C.; Austin,C. A.; Hamprecht, D. W.; Hartley, R. C. Tetrahedron Lett. (2004), 45, 8879–8882. |
| [44] | Bytschkov, I.; Doye, S.; Tetrahedron Lett. (2002), 43, 3715-3718. |
| [45] | Hisler, K.; Aurélien; Commeureuc, G. J. C.; Zhou, S.; Murphy, J. A.; Tetrahedron Lett. (2009), 50, 3290–3293. |
| [46] | Zhao,Y.C.; Zhang, J.; Wang, N.; Yu, H.; Yang, X. B.; Chen, S. Y.; Yu, X. Q.; Lett. in Org. Chemi. (2008), 5, 391-395. |
| [47] | Yu, H.; Zhang, J.; Zhao, Y. C.; Wang, N.; Wang, Q.; Yang, X. B.; Yu, X. Q.; Chem. Papers, (2008), 62, 187–193. |
| [48] | Yang, X. B.; Feng, J.; Zhang, J.; Wang,; Wang, L.; Liu, J. L.; Yu, X. Q, Org. Lett. (2008), 10, 1299-1302. |
| [49] | Majumder, S.; Gipson, K. R.; Staples, R. J.; Odom.; A. L. Adv. Synth. Catal. (2009), 351, 2013 – 2023. |
| [50] | Park, K. H.; Joo, H. S.; Ahn, K.; Jun, K.; Terahedron Lett. (1995), 36, 5943-5946. |
| [51] | Kassaee, M. Z.; Masrouri, H.; Movahedi, F.; Mohammadi, R. Helvetica Chimica Acta (2010), 93, 261-264. |
| [52] | Havaldar, H. F.; Mule, G.; Dabholkar, B.; Synth. Commun. (2011), 41, 2304–2308. |

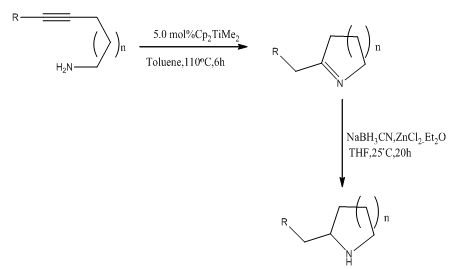
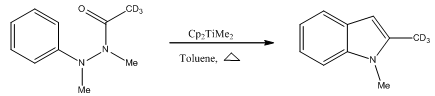
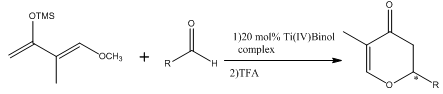
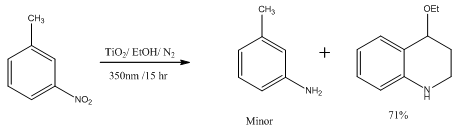
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML