-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(5): 122-126
doi: 10.5923/j.ajoc.20120205.03
Synthesis, Characterizations and Biological Screening of Tetrahydro-Quinazoline Analogues
Hiren Doshi 1, Maitreya Bhatt 1, Sampark Thakkar 2, Arabinda Ray 2
1Ashok & Rita Patel Institute of Integrated Study & Research in Biotechnology and Allied Sciences (ARIBAS), Sardar Patel University, NewV.V.Nagar-388121, Gujarat, India
2P. D. Patel Institute of Applied Sciences (PDPIAS), CHARUSAT, Changa-388421, Gujarat, India
Correspondence to: Hiren Doshi , Ashok & Rita Patel Institute of Integrated Study & Research in Biotechnology and Allied Sciences (ARIBAS), Sardar Patel University, NewV.V.Nagar-388121, Gujarat, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Quinazoline and their fused-ring systems are well known for their potential biological activity. In the present study a new Tetrahydro-quinazoline analogues (MB I-V) were synthesized. The newly synthesized compounds were characterized by IR, NMR and C, H, N, S analyses. All newly synthesized compounds were screened for their antibacterial (Pseudomonas aurigenosa Bacillus subtilis and Escherichia coli) studies. The results revealed that all synthesized compounds have a significant biological activity against the tested microorganisms.
Keywords: Tetrahydro-Quinazoline, Antibacterial Activity, Isophorone
Article Outline
1. Introduction
- During the ancient era the isolation of various compounds was done by the process of extraction. But this process was time consuming as well as laborious. Moreover the yield was very low and and the process of isolation required large amount of the starting material. Today the process of isolation has been replaced by the synthetic routes. A large number of compounds can be synthesized by using small amount of chemicals. More over the Synthetic routes take less amounts of time and can easily be carried out. Quinazoline derivatives hold a place of significant in todays world for their important application in chemical, clinical and biological spheres. Medicinally quinazoline has been used in various areas especially as an analgesic[1, 2], anti-oxidant[3-5], anti-cancer drugs[6-10],anti-inflammatory[11,12], anti-convulsant[13], anti-bacterial[14], anti-fungal[15] and anti-mycobacterial agents[16, 17]. It has also been found in the treatment of malaria[18, 19]. Considering the vast potential of quinazoline, it was thought appropriate to synthesized, characterized quinazoline analogues and investigates their biological activity. In this investigation, we have prepared tetrahydro quinazoline analogues and characterized them using spectral data. Biological screenings of these compounds were also reported also reported here.
2. Result and Discussion
2.1. Chemistry
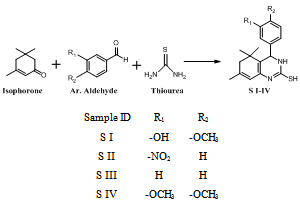
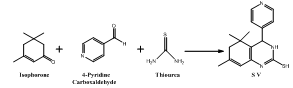
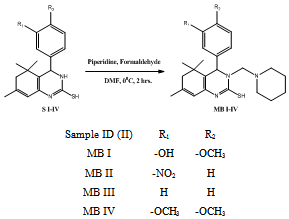
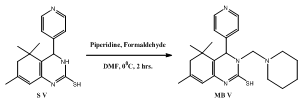
- Intermediates, 5,5,7-trimethyl-4-aryl-3,4,5,6-tetrahydro quinazoline -2-thiols (S I-V) synthesized from isophorone, different aromatic aldehydes and thiourea (Scheme Ia,b). Tetrahydroquinazoline analogues (MB I-V) synthesized from intermediates S I-V (Scheme IIa,b). The purity of the compounds was monitored by ascending thin layer chromatography (TLC) on silica gel-G coated on aluminum plates.Developing solvents used in TLC were ethyl acetate: hexane (1:2) and the plates were viewed under UV light 254 nm and 265 nm respectively. Melting points were determined by the open capillary tubes equipped in electro thermal melting point apparatus. All the melting points are uncorrected. The yields of all the compounds reported are of crystallized form. The elemental analysis was studied by C, H, N, S analyzer on Perkin Elmer (U.S.A, 2400 Series II). Infrared (IR) spectra were recorded for the compounds on Perkin Elmer Spectrum GX using KBr pellet disc technique. The structure will further be elucidated by recording its mass spectra on Shimadzu LCMS 2010 eV. NMR was recorded in Torrent pharmaceuticals Ltd Research centre on Bruker Avance FT- NMR 400 MHz.The M.P. of synthesized compounds were found between 258-300℃, while for the some analogues it was more than 300℃. The mol. wt. of the synthesized compound were in range of 271-427 g mole-1.
 | Scheme Ia |
 | Scheme Ib |
 | Scheme IIa |
 | Scheme IIb |
2.2. Antibacterial Activity
- In present study the five compounds are screen for antibacterial activity against gram positive and gram negative bacteria. The result of antibacterial activities are comparatively studied (Table 1) and are recorded on the basis of the presence or absence of inhibition zone on media plate. The antibacterial activity against Bacillus subtilis was seen in all the compounds (MB I-V) used. The compound MB I showed high activity against Bacillus subtilis due to presence of OH group. All the compounds (MB I-V) did not show activity on Pseudomonas aurigenosa as well as the standard drug did not show any activity. The compound MB II showed high activity against Escherichia coli. This could be due to the presence of -NO2 group at R1 position of tetrahydroquinazoline derivatives.
3. Experimental
- The raw materials used were of synthetic grade from Sigma Aldrich & Loba chemicals Ltd. Solvents used were distilled and dried. The TLC plates were from Merck. Melting points were determined by the open capillary tubes equipped reported are of crystallized form in electro thermal melting point apparatus. All the melting points are uncorrected. The elemental analysis was studied by C, H, N, S analyzer on Perkin Elmer (U.S.A, 2400 Series II). Infrared (IR) spectra were recorded for the compounds on Perkin Elmer Spectrum GX using KBr pellet disc technique. The structure will further be elucidated by recording its mass spectra on Shimadzu LCMS 2010 eV. NMR was recorded in Torrent pharmaceuticals Ltd Research centre on Bruker Avance FT- NMR 400 MHz.
3.1. General Procedure for Synthesis of 5, 5, 7-Trimethyl -4-Aryl-3, 4, 5, 6-Tetrahydroquinazoline-2-Thios (S I-V)
- Equivalent mixture of isophorone, an aromatic aldehydes and thiourea was refluxed in ethanol for 10 hours. Then the resulting reaction mixture was poured in ice bath. The obtain product (Scheme-Ia & Ib) was filtered, dried & recrystallized by 95% Ethanol[20].5-(2-mercapto-5,5,7-trimethyl-3,4,5,6-tetrahydroquinazolin-4-yl)-2-methoxyphenol (S I): Yield: 85%; Colour: Brown; m.p.: >300℃; IR (KBr, cm-1): 1462 (Ar C=C), 1598 (Ar C-C), 3403 (N-H), 3292 (O-H), 1365 (CH3), 1638 (C=N), 1219 (C-S), 1274 (Ester C-O); 1H NMR (DMSO): 1.25 δ (6H, s, CH3), 1.83 δ (3H, s, CH3), 2.19 δ (2H, s, CH2), 3.83 δ (3H, s, OCH3), 4.28 δ (1H, d, N-H), 4.60 δ (1H, d, CH), 5.35 δ (1H. s, OH), 5.7 δ (1H, s, CH), 6.3 δ (1H, s, S-H), 6.68 δ (1H, d, CH), 6.70 δ (1H, d, CH), 6.86 δ (1H, s, CH), DMSO 2.52 δ & 3.50 δ; MS, m/z: 317.34[M+1]. Anal.: Calc. for C, 65.42; H, 6.71; N, 8.48; S, 9.70. Found: C, 65.21; H, 6.50; N, 8.29; S, 9.35.5,5,7-trimethyl-4-(3-nitrophenyl)-3,4,5,6-tetrahydroquinazoline-2-thiol (S II): Yield: 78%; Colour: Brown; m.p.: >300ºC; IR (KBr, cm-1): 1664 (Ar C=C), 1468 (Ar C-C), 3383 (N-H), 3040 (Ar C-H), 1384 (CH3), 1619 (C=N), 1246 (C-S), 1528 (N-O); 1H NMR (DMSO): 1.26 δ (6H, s, CH3), 1.82 δ (3H, s, CH3), 1.98 δ (2H, s, CH2), 4.12 δ (1H, d, N-H), 4.59 δ (1H, d, CH), 5.61 δ (1H, s, CH), 5.8 δ (1H, s, S-H), 7.62 δ (1H, d, CH), 7.59 δ (1H, d, CH), 8.07 δ (1H, d, CH), 8.12 δ (1H, s, CH), DMSO 2.48 δ & 3.47 δ; MS, m/z: 329.23[M+1]. Anal.: Calc. for C, 61.98; H, 5.81; N, 12.76; S, 9.73. Found: C, 61.75; H, 5.75; N, 12.50; S, 9.60.5,5,7-trimethyl-4-phenyl-3,4,5,6-tetrahydroquinazoline-2-thiol (S III): Yield: 86%; Colour: Brown; m.p.: 258℃; IR (KBr, cm-1): IR (KBr, cm-1): 1663 (Ar C=C), 1452 (Ar C-C), 3438 (N-H), 2848 (Ar C-H), 1378 (CH3), 1617 (C=N) 1286 (C-S); 1H NMR (DMSO): 1.24 δ (6H, s, CH3), 1.83 δ (3H, s, CH3), 2.2 δ (2H, s, CH2), 4.01 δ (1H, d, N-H), 4.62 δ (1H, d, CH), 5.68 δ (1H, s, CH), 5.9 δ (1H, s, S-H), 7.25 δ (2H, t, CH), 7.28 δ (1H, t, CH), 7.34 δ (2H, t, CH), DMSO 2.52 δ & 3.53 δ; MS, m/z: 284.10[M+1]. Anal.: Calc. for C, 71.79; H, 7.09; N, 9.85; S, 11.27. Found: C, 71.50; H, 6.95; N, 9.67 S, 11.01.4-(3,4-dimethoxyphenyl)-5,5,7-trimethyl-3,4,5,6-tetrahydroquinazoline-2-thiol (S IV): Yield: 91%; Colour: Orange; m.p.: 285℃; IR (KBr, cm-1): 1652 (Ar C=C), 1462 (Ar C-C), 3192 (N-H), 2955 (Ar C-H), 1381 (CH3), 1612 (C=N), 1249 (C-S); 1H NMR (DMSO): 1.26 δ (6H, s, CH3), 1.82 δ (3H, s, CH3), 2.20 δ (2H, s, CH2), 3.88 δ (6H, s, OCH3), 4.11 δ (1H, d, N-H), 4.59 δ (1H, d, CH), 5.69 δ (1H, s, CH), 6.01 δ (1H, s, S-H), 6.76 δ (1H, d, CH), 6.67 δ (1H, d, CH), 6.73 δ (1H, s, CH), DMSO 2.5 δ & 3.51 δ; MS, m/z: 344.40[M+1]. Anal.: Calc. for C, 66.25; H, 7.02; N, 8.13; S, 9.31. Found: C, 65.99; H, 6.88; N, 8.04; S, 9.15.5,5,7-trimethyl-4-(pyridin-4-yl)-3,4,5,6-tetrahydroquinazoline-2-thiol (S V): Yield: 77%; Colour: Yellow; m.p.: 278ºC; IR (KBr, cm-1): 1667 (Ar C=C), 1467 (Ar C-C), 3406 (N-H), 2853 (Ar C-H), 1367 (CH3), 1599 (C=N), 1254 (C-S); 1H NMR (DMSO): 1.26 δ (6H, s, CH3), 1.82 δ (3H, s, CH3), 2.22 δ (2H, s, CH2), 4.19 δ (1H, d, N-H), 4.61 δ (1H, d, CH), 5.72 δ (1H, s, CH), 6.05 δ (1H, s, S-H), 7.35 δ (2H, d, CH), 8.55 δ (2H, d, CH), DMSO 2.51 δ & 3.53 δ; MS, m/z: 285.15[M+1]. Anal.: Calc. for C, 67.33; H, 6.71; N, 14.72; S, 11.23. Found: C, 67.28; H, 6.56; N, 14.51; S, 11.02.
3.2. General Procedure for Synthesis of 4-Aryl-5, 5-Dimethyl-7-(2’Piperidin-1’-Yl-Ethyl)-2-Thiol-3,4,5,6-Tetrahydroquinazolines (MB I-V)
- A mixture of 0.01 moles appropriate intermediate (S I-V), Piperidine & formaldehyde in DMF were stirred for 2 hours in cooling conditions. Then the resulting reaction mixture was poured in ice bath. The obtain product (Scheme-IIa & IIb) was filtered, dried & recrystallized by 95% Ethanol.5-(2-mercapto-5,5,7-trimethyl-3-(piperidin-1-ylmethyl)-3, 4, 5, 6 - tetrahydroquinazolin- 4- yl)-2-methoxyphenol (MB I): Yield: 82%; Colour: Brown; m.p.: 292ºC; IR (KBr, cm-1): 1652 (Ar C=C), 1417 (Ar C-C), 2894 (Ar C-H), 1061 (C-N), 3426 (N-H), 3035 (O-H), 1219 (Ester C-O), 1314 (CH3), 1265 (C-S), 1600 (C=N); 1H NMR (DMSO): 1.25 δ (6H, s, CH3), 1.28 δ (2H, m, CH2), 1.53 δ (4H, m, CH2), 1.83 δ (3H, s, CH3), 2.2 δ (2H, s, CH2), 2.45 δ (4H, m, CH2), 3.65 δ (2H, s, CH2), 3.84 δ (3H, s, OCH3), 4.59 δ (1H, d, CH), 5.33 δ (1H. s, OH), 5.70 δ (1H, s, CH), 6.32 δ (1H, s, S-H), 6.66 δ (1H, d, CH), 6.69 δ (1H, d, CH), 6.87 δ (1H, s, CH), DMSO 2.52 δ & 3.50 δ; MS, m/z: 427.24[M+1]. Anal.: Calc. for C, 67.41; H, 7.78; N, 9.83; S, 7.50. Found: C, 67.22; H, 7.52; N, 9.60; S, 7.25.5,5,7-trimethyl-4-(3-nitrophenyl)-3-(piperidin-1-ylmethyl)-3,4,5,6-tetrahydroquinazoline-2-thiol (MB II): Yield: 87%; Colour: Brown; m.p.: >300ºC; IR (KBr, cm-1): 1664 (Ar C=C), 1464 (Ar C-C), 2866 (Ar C-H), 1096 (C-N), 3404 (N-H), 1528 (N=O), 1382 (CH3), 1256 (C-S), 1620 (C=N); 1H NMR (DMSO): 1.25 δ (6H, s, CH3), 1.29 δ (2H, m, CH2), 1.52 δ (4H, m, CH2), 1.82 δ (3H, s, CH3), 2.0 δ (2H, s, CH2), 2.45 δ (4H, m, CH2), 3.60 δ (2H, s, CH2), 4.62 δ (1H, d, CH), 5.64 δ (1H, s, CH), 5.84 δ (1H, s, S-H), 7.58 δ (1H, d, CH), 7.63 δ (1H, d, CH), 8.09 δ (1H, d, CH), 8.13 δ (1H, s, CH), DMSO 2.51 δ & 3.5 δ; MS, m/z: 426.21[M+1]. Anal.: Calc. for C, 64.76; H, 7.09; N, 13.13; S, 7.52. Found: C, 64.52; H, 6.89; N, 12.96; S, 7.36.5,5,7-trimethyl-4-phenyl-3-(piperidin-1-ylmethyl)-3,4,5,6-tetrahydroquinazoline-2-thiol (MB III): Yield: 79%; Colour: Brown; m.p.: 277℃; IR (KBr, cm-1): 1660 (Ar C=C), 1423 (Ar C-C), 2885 (Ar C-H), 1077 (C-N), 3420 (N-H), 1370 (CH3), 1248 (C-S), 1596 (C=N); 1H NMR (DMSO): 1.25 δ (6H, s, CH3), 1.27 δ (2H, m, CH2), 1.53 δ (4H, m, CH2), 1.82 δ (3H, s, CH3), 2.19 δ (2H, s, CH2), 2.44 δ (4H, m, CH2), 3.63 δ (2H, s, CH2), 4.61 δ (1H, d, CH), 5.7 δ (1H, s, CH), 5.97 δ (1H, s, S-H), 7.25 δ (1H, t, CH), 7.27 δ (2H, t, CH), 7.35 δ (2H, t, CH), DMSO 2.5 δ & 3.51 δ; MS, m/z: 381.22[M+1]. Anal.: Calc. for C, 72.40; H, 8.19; N, 11.01; S, 8.40. Found: C, 72.15; H, 8.09; N, 10.78; S, 8.19.4-(3,4-dimethoxyphenyl)-5,5,7-trimethyl-3-(piperidin-1-ylmethyl)-3,4,5,6-tetrahydroquinazoline-2-thiol (MB IV): Yield: 65%; Colour: Orange; m.p.: >300℃; IR (KBr, cm-1): 1646 (Ar C=C), 1425 (Ar C-C), 2858 (Ar C-H), 1068 (C-N), 3443 (N-H), 1227 (Ester C-O), 1360 (CH3), 1258 (C-S), 1629 (C=N); 1H NMR (DMSO): 1.28 δ (6H, s, CH3), 1.53 δ (2H, m, CH2), 1.59 δ (4H, m, CH2), 1.83 δ (3H, s, CH3), 2.21 δ (2H, s, CH2), 2.23 δ (4H, m, CH2), 3.61 δ (2H, s, CH2), 3.85 δ (6H, s, OCH3), 4.60 δ (1H, d, CH), 5.22 δ (1H, s, CH), 6.06 δ (1H, s, S-H), 6.67 δ (1H, d, CH), 7.73 δ (1H, d, CH), 7.76 δ (1H, s, CH), DMSO 2.50 δ & 3.52 δ; MS, m/z: 428.81[M+1]. Anal.: Calc. for C, 67.99; H, 7.99; N, 9.51; S, 7.26. Found: C, 67.65; H, 7.72; N, 9.26; S, 7.01.5,5,7-trimethyl-3-(piperidin-1-ylmethyl)-4-(pyridin-4-yl)-3,4,5,6-tetrahydroquinazoline-2-thiol (MB V): Yield: 78%; Colour: Yellow; m.p.: 268℃; IR (KBr, cm-1): 1653 (Ar C=C), 1418 (Ar C-C), 2894 (Ar C-H), 1094 (C-N), 3441 (N-H), 1368 (CH3), 1265 (C-S), 1600 (C=N); 1H NMR (DMSO): 1.25 δ (6H, s, CH3), 1.28 δ (2H, m, CH2), 1.53 δ (4H, m, CH2), 1.8 δ (3H, s, CH3), 2.21 δ (2H, s, CH2), 2.44 δ (4H, m, CH2), 3.62 δ (2H, s, CH2), 4.59 δ (1H, d, CH), 5.71 δ (1H, s, CH), 6.08 δ (1H, s, S-H), 7.36 δ (2H, d, CH), 8.54 δ (2H, d, CH), DMSO 2.52 δ & 3.51 δ; MS, m/z: 382.22[M+1]. Anal.: Calc. for C, 69.07; H, 7.90; N, 14.65; S, 8.38. Found: C, 68.92; H, 7.76; N, 14.49; S, 8.13.
3.3. Antibacterial Study
- Culture of microorganisms: Bacterial samples used for primary screening of antibacterial activity for the compounds. Bacteria strains were supplied from ARIBAS, namely Bacillus subtilis, Pseudomonas aurigenosa, Escherichia coli. They were maintained by periodical transfer on freshnutrient agar slant.Preparation of media: The antibacterial activities were carried out by using Mueller-Hinton agar plate. The composition of the medium was Nutrient broth 13g & Agar-Agar powder 30 g. Both ingredients were completely dissolved in 1 L of distilled water & the pH of the medium was adjusted to 7.4 pH. The medium was sterilized in an autoclave at 121℃ for 15 min. It was then cooled down to 45℃ & 20 ml was poured in each sterilized Petri dish.Preparation of inoculums: A fresh microbial seed was prepared separately by sub culturing in to nutrient broth medium and incubated at 37℃.Antibacterial screening test: The antibacterial activity was performed after the checking of minimum inhibitory concentration (MIC) by taking (0.04g/mL) of sample using the cup method. Minimum inhibitory concentration is the lowest substance concentration at which no sign of bacterial growth was detectable microscopically. The test compounds were dissolved in DMSO to produce (0.04g/mL) for minimum inhibitory concentration (MIC). Different compounds on different microorganisms were placed in each cup. The plates were first placed in a refrigerator for 30 minutes and incubated at 37ºC for 24 hours. The results were recorded by measuring the zone of inhibition in the plate and recorded for further calculation.
4. Conclusions
- The antibacterial; activity of the compound MB I to MB V was screened against the three different types of bacterial strains (P. aurigenosa, E. coli, B. subtilis). None of these compound was found to be effective against P. aurigenosa However all these compounds are active against B. subtilis and E. coli compare to standard i.e. ciprofloxacin, the compound reported here are comparable against E. coli. However, all the compounds have very good activity against the bacterial strain, it is interesting to note that MB I and MB II are the most potent agents, have OH and NO2 group attached to aromatic ring respectively.
ACKNOWLEDGEMENTS
- We thank Charutar Vidya Mandal, New VallabhVidyanagar for providing facility and financial support to undertake the research work. The authors are also gratefullyacknowledged the support of Torrent pharmaceuticals Ltd Research centre, Ahmedabad, Gujarat, India.
References
| [1] | Amin K M, Kamel M M, and Anwar M M. European Journal of Medicinal Chemistry. 45, 2117–2131, 2010. |
| [2] | Alagarsamy V, Raja Solomon V, Dhanabal K. Bioorganic & Medicinal Chemistry. 15, 235–241, 2007. |
| [3] | Vijaynathappa J, Bhojraj S. Journal of health sciences. 54, 524-528, 2008. |
| [4] | Gaur K, Kori M.L. Academic J. of Plant Sci. 2, 60-64, 2009. |
| [5] | Kerri, Rao M N A. Oxygen radical scavenging activity of curcumin, Int.J.pharma. 58, 237-240, 1990. |
| [6] | Manihandrika P, Sridhar V. Indian journal of chemistry. 48B, 840-847, 2009. |
| [7] | Al-Obaid A M, Abdel-Hamide S G, El-Kashef H A. European Journal of Medicinal Chemistry. 44, 2379–2391, 2009. |
| [8] | Chandregowda V, Kush A.K, Chandrasekara Reddy G. European Journal of Medicinal Chemistry. 44, 3046-55, 2009. |
| [9] | Giri R S, Thaker H M, Giordano T. Bioorganic & Medicinal Chemistry. 18, 2796–2808, 2010. |
| [10] | Qian L, Shen Y, Chen J. Acta Phys. -Chim. Sin. 22, 1372−1376, 2006. |
| [11] | Giri R S, Thaker H M, Giordano T. European Journal of Medicinal Chemistry. 44, 2184–2189, 2009. |
| [12] | Laddha S S, Bhatnagar S P. Bioorg. & Med. Chem.. 17, 6796–02, 2009. |
| [13] | Jatav V, Mishra P, Kashaw S, Stables J P. European Journal of Medicinal Chemistry. 43, 1945-1954, 2008. |
| [14] | Vachala D, Unnissa H. Indian journal of heterocyclic chemistry. 17, 347-350, 2008. |
| [15] | Guang-Fang Xu, Bao-An Song, Bhadury P S. Bioo ganic & Medicinal Chemistry. 15, 3768–3774, 2007. |
| [16] | Kabri Y, Nadine A, Dume tre A L. European Journa of Medicinal Chemistry. 45, 616–622, 2010. |
| [17] | Subramaniam A, Faaleolea E R, Goldman R C, Tuberculosis. 89, 334–353, 2009. |
| [18] | Ashraf A. Khalil, Sami G. Abdel Hamide, Abdulrahman M. Al-Obaid, Hussein I. El-Subbagh; Substituted Quinazolines, Part 2. Synthesis and In-Vitro Anticancer Evaluation of New 2-Substituted Mercapto-3H-quinazoline Analogs; Arch. Pharm. Pharm. Med. Chem., 2, 95–103, 2003. |
| [19] | Guan J, Zhang Q, O'Neil M, Obaldia N 3rd, Ager A, Gerena L, Lin AJ. Antimalarial activities of new pyrrolo[3, 2-f] quinazoline-1, 3-diamine derivatives. Antimicrob Agents Chemother. Dec; 49(12):4928-33, 2005. |
| [20] | Nakamoto K. IR of inorganic and coordination compound, John Willey & Sons, pp 54, 1963. |
| [21] | Honkanen E, Pipuri A, Kairisalo P, Nore P, Karppaness H and Paakari I, J. Med. Chem.; 26; 143, 1983. |
| [22] | Saundane A. Rudresh K. Satyanarayan N. Hiremath S. Pharmacological screening of 6H, 11H-Indolo {3, 2-C} isoquinolin-5-ones & their derivatives. J Ind pharm sci., 60, 379-383, 1998. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML