-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(5): 116-121
doi: 10.5923/j.ajoc.20120205.02
Green Thiocyanation of Aromatic and Heteroaromatic Compounds by Using Silica Boron Sulfonic Acid as a New Catalyst and H2O2 as Mild Oxidant
Sami Sajjadifar
Department of Chemistry, Faculty of Science, Ilam University, P.O. Box 69315516, Ilam, Iran and Department of Chemistry, Payame Noor University, PO BOX 19395-4697, Tehran, Iran
Correspondence to: Sami Sajjadifar , Department of Chemistry, Faculty of Science, Ilam University, P.O. Box 69315516, Ilam, Iran and Department of Chemistry, Payame Noor University, PO BOX 19395-4697, Tehran, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Highly efficient regioselective thiocyanations of indoles and N,N-disubstituted anilines are achieved via a green and simple protocol using Silica Boron Sulfonic Acid (SBSA) as a new catalyst, KSCN and hydrogen peroxide as a mild and environmentally friendly oxidant in aqueous condition.
Keywords: Silica Boron Sulfonic Acid, SBSA, BSA, Thiocyanation, Green Synthesis, Hydrogen Peroxide
Article Outline
1. Introduction
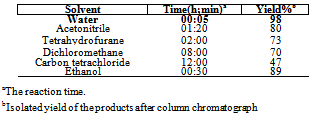
- Thiocyanates play an important role in organic synthesis[1] and in pharmaceuticals[2]. Thiocyanate is a versatile synthon which can be readily transferred to other functional groups such as sulfide[3], aryl nitrile[4], thiocarbamate[5], and thionitrile[6]. Therefore, it is important to find the new and fast methods for synthesis of thiocyanate group containing aromatic systems[7]. In view of the versatility of thiocyanate group in heterocyclic construction[8], it will be of significance to probe the thiocyanation of aromatic and heteroaromatic compounds. Several methods have been developed for the thiocyanation of arenes by using various reagents under certain conditions[9-18]. Yet, only a limited number of reagents, such as bromine/potassium thiocyanate (only for indoles)[15], N-thiocyanatosuccinimide (only for 5- methoxy-2-methylindole and accompanied by two bisthiocyanates)[16], ceric ammonium nitrate (CAN)[17], acidic mont K10 clay[18], iodine/methanol, oxone[19], diethyl azodicarboxylate[20], IL-OPPh2[21], potassium peroxydisulfate–copper(II)[22], have been applied to the thiocyanation of aromatic and heteroaromatic systems. However, these methodologies suffer from one or more drawbacks such as the less availability or hard preparation of starting materials[15-16], the requirement for a large excess of strong oxidizing reagents, low yields for some compounds[17], and performances under certain special conditions[18]. Hence, a requirement for developing alternative synthesis routines accessible to the thiocyanation of aromatic and heteroaromatic compounds is in high demand. Avoiding organic volatile solvents during the reactions in organic synthesis leads to a clean, efficient, and economical technology.There is an increasing interest in the use of environmentally benign reagents and procedures. In other words, the presence of green solvent coupled with the high yields and short reaction times often associated with reactions of this type make these procedures very attractive for synthesis[23]. Aqueous mediated reactions have received considerable attention in organic synthesis due to the environmental safety reasons. Water is a desirable solvent for chemical reactions because it is safe, non-toxic, environmentally friendly, readily available, and inexpensive compared to organic solvents[24-26]. It has high dielectric constant and cohesive energy density compared to organic solvents. It has also special effects on reactions arising from inter and intramolecular non-covalent interactions leading to novel solvation and assembly processes. Water as a reaction medium has been utilized for large numbers of organic reactions[27]. Thus, the development of an efficient and convenient synthetic methodology in aqueous medium is an important area of research[28-29]. However, most of the catalysts are expensive and may lead to the environmental pollution. With the overgrowth of environmental and economic concerns, the development of benign catalytic processes for organic reaction is becoming increasingly important[30]. The solvent effect on product yields was investigated using 1a as a substrate. Both the yields and reaction times listed in Table 1 suggest that water appears to be very favorable for thiocyanations in presence of SBSA (as a strong and new catalyst).
|
|
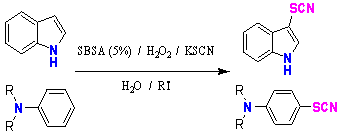 | Scheme 1. Thiocyanation reaction by using SBSA as a new catalyst |
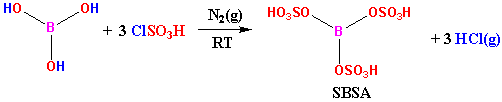 | Scheme 2. Production of SBSA |
2. Methods
2.1. General
- Chemicals were purchased from Merck chemical company. Yields refer to isolated products.Thiocynation of indole to 3-thiocyanato indole. A typical procedure: A suspension of indole (0.117 g, 1 mmol), potassium thiocyanate (0.294 g, 3 mmol), SBSA (0.05 g, 5%) in H2O (7-10 mL) was stirred at room temperature for 5-10 min. then H2O2 (0.45mL) was added dropwise (2-5 min). The progress of the reaction was monitored by TLC (ethylacetate: n-hexane 1:10). After completion of the reaction, the reaction mixture was decanted with CHCl3 (3×20 mL). Anhydrous Na2SO4 (5 g) was added to the organic layer and filtered off after 20 min. Chloform was removed. The yield was 0.167 g, (96%), dark brown solid mp 71-73 ℃(1b)[2,19,37]. FT-IR (KBr): 2159, 3289, 1H-NMR (FT-300 MHz, CDCl3/TMS): dppm 8.87 (1H, br s, NH), 7.83 (1H, d, J= 8.8 Hz), 7.46–7.23 (4H, m). 13C NMR (75 MHz, CDCl3): 136.06, 131.22, 127.66, 123.83, 121.87, 118.65, 112.24, 91.76.
2.2. Preparation of Silica Boron Sulfonic Acid (SBSA)[36]
- A 50 mL suction flask was equipped with a constant pressure dropping funnel. The gas outlet was connected to a vacuum system through an adsorbing solution (water) and an alkali trap. Boric acid (1.55 g, 25 mmol) was charged in the flask and chlorosulfonic acid (8.74 g, ca. 5 mL, 75 mmol in 5 ml CH2Cl2) was added dropwise over a period of 1 h at room temperature under N2 gas. HCl evolved immediately. After completion of the addition, the mixture was shaken for 85 min, while the residual HCl was eliminated by suction. Then the mixture was washed with diethyl ether to remove the unreacted chlorosulfonic acid and then add 14.4 g SiO2 and mix. Finally, dried and a grayish solid material was obtained in 95.66% yield (21.6g). 1HNMR of BSA in Acetone-D6 show δ=12.218.
2.3. Selected Specteral Data
2.3.1. 1-Methyl-3-thiocyanato Indole(2b)[2,19,37]
- White brown solid m.p 76-78℃ FT-IR (kbr): 2146 cm-1, 1H-NMR (FT-300 mhz, cdcl3/TMS): dppm 7.84-7.36 (5H, m), 3.74 (3H, s). 13C NMR (75 MHz, CDCl3): 137.17, 135.22, 128.47, 123.45, 121.62, 118.88, 112.106, 110.36, 33.42.
2.3.2. 2-Methyl-3-thiocyanato Indole(3b)[2,19,37]
- Brown solid, mp 104–106℃, FT-IR (kbr): 2151 cm-1, 3395cm-1, 1H-NMR (FT-300 MHz, CDCl3/TMS): dppm 8.65 (1H, br s, NH), 7.71 (1H, d, J = 8.8 Hz), 7.33–7.23 (3H, m), 2.5 (3H, s). 13C NMR (75 MHz, CDCl3): 142.152, 135.14, 128.69, 122.96, 121.55, 118.04, 111.30, 88.72, 12.05.
2.3.3. 5-Bromo-3-thiocyanato Indole(4b)[2,19,37]
- White brown solid mp 114-116 ℃, FT-IR (KBr): 2146, 3351cm-1, 1H-NMR (FT-300 MHz, CDCl3/TMS): dppm 8.87 (1H, br s, NH), 7.90- 7.15 (5H, m), 13C NMR (75 MHz, CDCl3): 134.68, 132.29, 129.32, 123.15, 121.27, 115.37, 113.72, 111.98, 102.212.
2.3.4. N1,N1,N8,N8-Tetramethyl-4-thiocyanato Naphthalene-1,8-Diamine(5b)
- Gray solid, m.p 79-81℃, FT-IR (kbr): 2055cm-1, 1H-NMR (FT-300 MHz, CDCl3/TMS): dppm 7.97-7.66 (5H, m,), 3.37 (12H, s). 13C NMR (75 MHz, CDCl3): 148.23, 144.64, 135.14, 132.6, 129.36, 127.12, 121.38, 120.7, 118.04, 115.4, 111.30, 46.97.
2.3.5. 4-Thiocyanato N-phenyl Morpholine(6b)
- Brown solid mp 75-77 ℃, FT-IR (KBr): 2156 cm-1. 1H-NMR (FT-300 MHz, CDCl3/TMS): dppm 7.48-7.40 (2H, d, J=8.4), 6.92-6.85 (2H, d, J=8.4), 3.88-3.85 (t, 4H, t, J=4.5), 3.24-3.21 (4H, t, J=4.5). 13C NMR (75 MHz, CDCl3): 152.54, 133.78, 116.19, 111.97, 111.17, 67.15, 48.06.
2.3.6. 4-Thiocyanato N-phenyl-2,2-iminodiethanol(7b)
- Dark brown solid mp 67-69℃, FT-IR (kbr): 2153, 3300 cm-1 1H-NMR (FT-300 mhz, cdcl3/TMS): dppm 7.43-7.33 (2H, d, J=7.2), 6.70-6.61 (2H, d, J=7.2), 3.75-3.60 (4H, m), 3.55-3.35 (4H, m), 2.15 (2H, s). 13C NMR (75 MHz, CDCl3): 151.8, 132.1, 116.5, 114.3, 111.7, 58.72, 55.9.
2.3.7. 4-Thiocyanato N-phenyl 15-crown-5(8b)
- Brown solid, m.p 78-80℃ FT-IR (kbr): 2147, 1094 cm-1. 1H-NMR (FT-300 MHz, CDCl3/TMS): dppm 7.35-7.37 (2H, d J=8.2), 6.64-6.62 (d, 2H, J=8.2), 3.57-3.72 (20H, m). 13C NMR (75 MHz, CDCl3): 149.32, 134.77, 112.80, 105.94, 112.65, 71.26, 70.22, 69.98, 68.01, 52.67.
2.3.8. 4-Thiocyanato N,N-dimethyl Aniline(9b)[2,19,37]
- Dark brown solid, mp 72-73℃, FT-IR (kbr): 2146 cm-1, 1H-NMR (FT-300 mhz, cdcl3/TMS): dppm 7.44-7.41(2H, d, J=8.8), 6.69-6.67 (2H, d, J=8.8), 3.007 (6H, s). 13C NMR (75 MHz, CDCl3): 151.67, 134.52, 113.13, 112.702, 106.37, 40.16.
2.3.9. 4-Thiocyanato N,N-diethyl Aniline(10b)[2,19,37]
- Brown-red Liquid, FT-IR (KBr): 2151 cm-1 1H-NMR (FT-300 MHz, CDCl3/TMS): dppm 7.5-7.15 (4H), 3.43 (4H, q), 1.14 (6H, t). 13C NMR (75 MHz, CDCl3): 149.65, 134.97, 125.28, 121.78, 112.68, 44.56, 12.30.
3. Results and Discussion
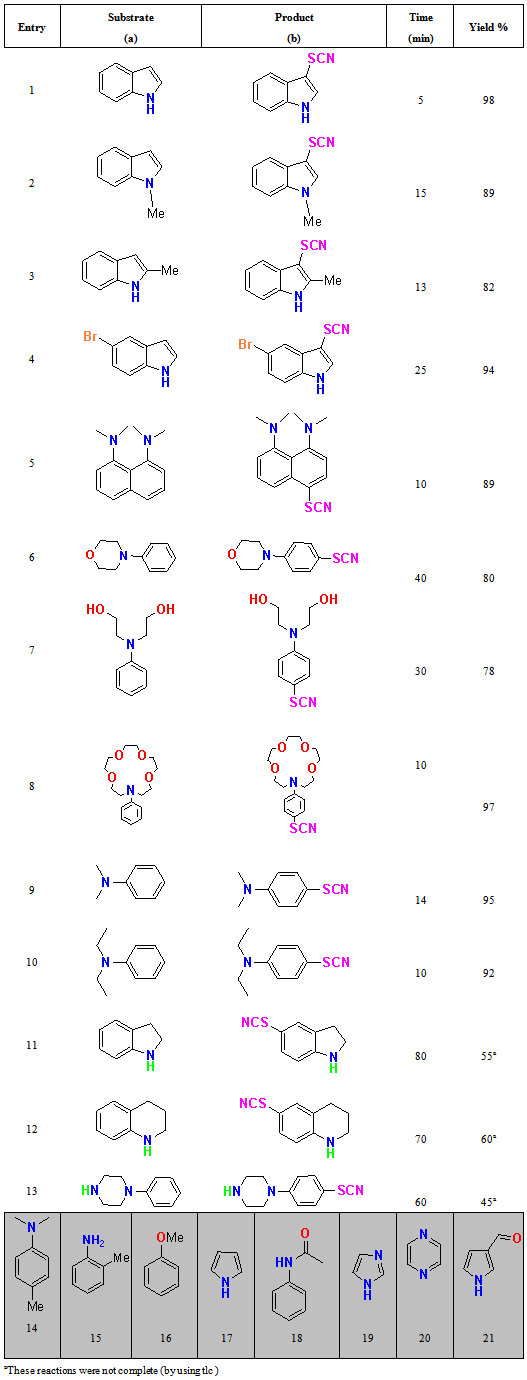
- In order to find a suitable catalyst for thiocyanation reaction in presence of H2O2 as a mild oxidant[38-39] from indole was chosen as a model to provide compound 1b (Scheme 4), and its behavior was studied in various solvents at room temperature. For chosen better solvent, different solvents were examined (Table 1). Water is a desirable solvent for chemical reactions for reasons of cost, safety and environmental concerns, use of water in this reaction gave good yields of products. The optimum yields of the products are obtained when 5 mol% of SBSA is used. Total reactions are summarized in Table 2. The thiocyanation was investigated in various conditions. In the absence of SBSA, reaction was not accomplished, but in the presence of SBSA 5% (0.05 g SBSA equal 0.15 mmol H+) the reaction took place with best result. The stoichiometry of the reactants was also varied. A ratio of 1:3:4 (indole: KSCN: H2O2) was found to be the most suitable, and decreasing the amount of H2O2 or potassium thiocyanate increased the reaction time and lowered the yield. The substrate scope of this reaction was then examined using various arenes under the optimized conditions (Table 2). In general, this reaction provided good-to-excellent yields with all the substrates tested. As shown in Table 2, indole and electron-rich indoles gave the desired products in excellent yields (Table 2, entries 1-3). Also, electron- deficient indoles such as 5- bromoindole reacted with potassium thiocyanate and SBSA/H2O2 to afford the corresponding 5-bromo-3- thiocyanato indole in good yield, but required longer reaction time (Table 2, entry 4). This observation can be attributed to the lower electron density of such substrates. The lower yield is probably attributed to the steric hindrance of 2- substituted indole (Table 2, entries 3). The addition was highly regioselective occurring at the 3-position of the indole ring.19,37 Various N,N-disubstituted aromatic amines were converted into the respective 4-thiocyanato amine in high to excellent overall yields (Table 2, entries 5–10). The reactions were clean and the products were obtained with high para-selectivity (Table 2, entry 9, 10). However, in the case of a parasubstituted amine, ortho thiocyanation did not occur (Table 2, 14). When indoline was used as a substrate, the reacion was not complete (Table 2, entry 11). The same result was shown for 1,2,3,4 tetrahydroquinoline(Table 2, entry 12) and 1- phenylpiperazine (Table 2, entry 13). On the other hand, N,N,4-trimethylaniline, o-methylaniline,anisole, pyrole, acetanilide, imidazole, pyrazine and 3-carbaldehydepyrole (Table 2, 14-21) did not react with potassium thiocyanate and SBSA/H2O2 to afford the corresponding derivatives. In comparison with other reported methods using other reagents which require refluxing conditions, the assistance of ultrasonic irradiation, toxic solvent or oxidant, this method works under milder and green reaction conditions. A proposed reaction mechanism is shown in scheme 3. In the first step, H2O2 reacts with SBSA (H+) to produce in situ hydrogen peroxonium ion (H3O2+). We have shown that the counter ion of acid did not have any role in the course of the reaction so that H2SO4 acts the same as HCl. Subsequently, reaction of this cation with SCN generates HOSCN, which in the presence of H+ is able to produce thiocyanium ion (S-CN)+ and H2O. In the last step electrophilic substitution of thiocyanium ion (SCN)+ with indole will generate the corresponding indolethiocyanate.
4. Conclusions
- We have found that SBSA is also active as a new catalyst towards the thiocyanation of aromatic compounds using H2O2 as oxidant (Scheme 1). The protocol reported here is mild and efficient for the thiocyanation of aromatic and heteroaromatic compounds. The mild reaction conditions, environmentally friendly oxidant and solvent, short reaction time and low cost are the obvious advantages of the present method. To identify new conditions for the synthesis of aryl thiocyanates, we began with an investigation of the conversion of indole into the corresponding indole thiocyanate using SBSA/H2O2/KSCN in the water as a model reaction (Scheme 4).
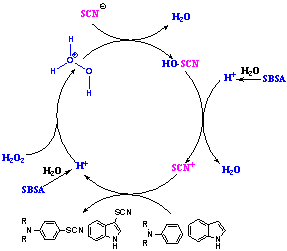 | Scheme 3. Proposed mechanism for the thiocyanation of indole and N,N-dialkylaniline |
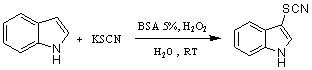 | Scheme 4. Indole thiocyanation |
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge partial support of this work by Payame Noor University (PNU) of Ilam.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML