-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(3): 52-57
doi: 10.5923/j.ajoc.20120203.03
Synthesis of Novel Azo Disperse dyes Derived from 4-Aminoantipyrine and their Applications to Polyester Fabrics
Ahmed A. Fadda , Khaled M. Elattar
Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
Correspondence to: Khaled M. Elattar , Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Nine variously substituted azo dyes derivatives 2-10 of antipyrine were prepared. The effects of the nature and orientation of the substituents on the color and dyeing properties of these dyes on polyester fibres were evaluated. The newly synthesized compounds were characterized by elemental analyses and spectral data (IR, 1H NMR, 13C-NMR and MS). The investigated dyes were applied to polyester fabrics and showed good light, washing, heat and acid perspiration fastness. The remarkable degree of brightness after washings is indicative of good penetration and the excellent affinity of these dyes for the fabric. The results in general revealed the efficiency of the prepared compounds as new azo dyes.
Keywords: 4-Aminoantipyrine, Enaminonitriles, Azo Disperse Dyes, Dyeing, Polyester Fibres
Article Outline
1. Introduction
- In recent years, there has been increasing interest in syntheses of heterocyclic compounds that have biological and commercial importance. Antipyrine compounds play an important role in modern organic synthesis, not only because they constitute a particularly useful class of heterocyclic compounds[1-3], but also because they are of great biological interest. They have been found to have biological[4], clinical[5], and pharmacological[6, 7], activities. One of the most important derivatives of antipyrine is 4-amino- antipyrine, which is used as a synthetic intermediate to prepare polyfunctionally substituted heterocyclic moieties with anticipated biological activity[8], analgesic[9, 10], anti- inflammatory[10], antimicrobial[11-13], and anticancer[14], activities. It was of interest to study the reactivity of antipyrinylhydrazonomalononitrile towards different nitrogen nucleophiles as well as activated nitriles.Considerable studies have been devoted to azo dyes derived from 4-aminoantipyrine[15-19]. Fadda et al[20-24], have been reported the synthesis of different azo disperse dyes for synthetic fibres. Recently, other studies reported the application of synthesized azo dyes to polyester fabrics[25-27]. Thus, we have initiated a program of applying the synthesized dyes derived from 4-aminoantipyrine to polyester as disperse dyes to study their colour measurement and fastness properties. We aim to synthesize a series of new dyes derived from 4-aminoantipyrine to apply these new dyes to polyester fabrics with the hope to get excellent fastness results.
2. Results and Discussion
2.1. Chemistry
- The synthetic strategies adopted to obtain the target compounds are depicted in Scheme 1. Diazonium salt of 4-aminoantipyrine undergo a coupling reaction with malononitrile in ethanolic sodium acetate solution at 0-5oC to give hydrazonomalononitrile derivative 2[28]. Compound 2 reacted with different secondary aminesnamely;[piperidine, morpholine, piperazine, pyrrolidine, diphenyl amine, ethyl 2-(4-chlorophenylamino)acetate, N- methylglucamine and 1-phenylpiperazine] in refluxing ethanol to afford the corresponding 1:1 acyclic enaminonitrile adducts 3-10, respectively. The formation of enaminonitriles 3-10 was illustrated through the initial addition of the secondary amines to cyano function to form the imino form followed by[1, 5]H migration to form the enamine form. The general structural formula for dyes 2-10 is as shown in Scheme 1. The structures of enaminonitriles 3-10 were confirmed by elemental analyses and spectral data. The IR spectra exhibited absorption bands due to stretching vibrations of NH2 group within υ = 3450-3301 cm-1, within υ = 2186-2171 cm-1 due to CN function and within υ = 1648-1610 cm-1 due to carbonyl groups. The 1H-NMR spectrum of compound 3 revealed the presence of three multiplet signals at δ 1.58-1.69, 3.52-3.62 and 7.31-7.52 ppm attributable to (3CH2, piperidine), (2CH2, piperidine) and aromatic protons, respectively, revealed two singlet signals at δ 2.63 and 3.16 ppm due to methyl and N-methyl protons, respectively and amino protons appeared at δ 7.13 ppm as broad singlet signal. The 13C-NMR spectra revealed signals due to cyano group within δ = 114.8-114.3 ppm. Furthermore, the detailed 1H-NMR and 13C-NMR spectra for each compound were mentioned in the experimental section. Moreover, the mass spectroscopic measurement of compounds 3-5 and 8-10 showed the molecular ion peaks at 367 (M+, 12.3), 368 (M+-1, 6.7), 477 (M+, 100.0), 495 (M+, 17.5), 368 (M+, 11.4) and 444 (M+, 5.0), respectively, which are equivalent with the molecular formula of the structures.However, no details regarding the dyeing behaviour of these compounds as disperse dyes for dyeing polyester fibres have been reported.
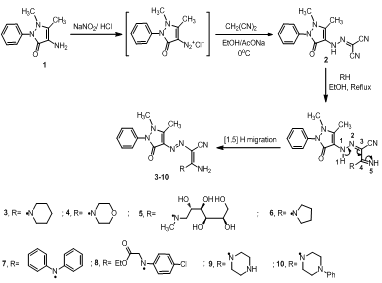 | Scheme 1. Synthetic route for the preparation of acyclic enaminonitriles 3-10 |
2.2. Dyeing of Polyester Fabrics and Dyeing Properties
2.2.1. Colour Measurement
- The effect of nature of different substituents on dyeing behaviour, colour hue and depth was discussed. This investigation depends on some spectral data of the dyed materials. The most commonly used function f(R) is the one developed theoretically by Kubelka and Munk. In their theory, the optical properties of a sample are described by two values "K" is the measure of the light absorption and "S" is a measure of the light scattering. On textiles, "K" is determined primarily by the dyestuffs and "S" only by the substrate. From the wave length Kubelka and Munk calculate the following relationship for reflectance R of thick, opaque sample with the constant of "K"and
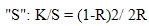 | (1) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- The negative sign of ΔC indicates that the dyed fiber becomes duller than the standard but, a positive sign indicates that the dyed fiber become brighter than the standard. The negative sign of ΔH indicates that the colour directed to red colour while, a positive sign indicates that the colour directed to yellowish.The values of K/S of compounds 2-10 vary from 0.43 to 2.70. The introduction of N-methylglucamine, pyrrolidine, piperazine and N-phenyl piperazine moieties in dyes 5, 6, 9 and 10, respectively increase the strength of K/S value and deepens the colour compared with the parent dye 2 (Table 1).All dyes with (+ve ΔC) values and are brighter than the parent dye 2. All dyes with (-ve ΔL) values and are darker than the parent dye 2. The positive value of a* and b* indicates that all group shift the colour hues of the dye to reddish direction on the red-green axis and to yellowish direction in the yellow-blue axis, respectively.
2.2.2. Assessment of Colour Fastness
- Most influences that can affect fastness are light, washing, heat and perspiration and atmospheric pollution. Conditions of such tests are chosen to correspond closely to treatments employed in manufacture and of ordinary use conditions[30]. Results are given after usual matching of tested samples against standard reference (the grey scale)[30]. The results revealed that these dyes have good fastness properties (Table 2).
3. Conclusion
- Newly synthesized azo dyes incorporated antipyrine moiety seems to be interesting for application to polyester fabrics. Furthermore, optical measurements and fastness properties were investigated. Nine useful disperse dyes 2-10 were synthesized by diazo coupling of 4-aminoantipyrine with malononitrile followed by addition of different secondary amines to the obtained coupling product. The dyes 2-10 were investigated for their dyeing characteristic on polyester and showed good light, washing, heat and acid perspiration fastness. The remarkable degree of brightness after washings is indicative of good penetration and the excellent affinity of these dyes for the fabric due to the accumulation of polar groups. The results in general revealed the efficiency of the prepared compounds as new azo dyes.
4. Experimental
4.1. Synthesis
- All melting points are recorded on Gallenkamp electric melting point apparatus. The IR spectra υ cm-1 (KBr) were on Perkin Elmer Infrared Spectrophotometer Model 157, Grating. The 13C-NMR and 1H-NMR spectra were run on Varian Spectrophotometer at 100 and 400 MHz, respectively, using tetramethylsilane (TMS) as an internal reference and using dimethylsulfoxide (DMSO-d6) as solvent. The mass spectra (EI) were run at 70 eV with JEOL JMS600 equipment and/or a Varian MAT 311 A Spectrometer. Elemental analyses (C, H and N) were carried out at the Microanalytical Center of Cairo University, Giza, Egypt. The results were found to be in good agreement with the calculated values. 4-Amino antipyrine (1) (mp 106-110oC) was purchased from Aldrich Company. The dyeing assessment, fastness tests, color measurements were carried out in El-Nasr Company for Spinning and Weaving El-Mahalla El-Kubra, Egypt.Synthesis of 2-[(1,5-dimethyl-3-oxo-2-phenyl-2, 3- dihydro-1H-pyrazol-4-yl)-hydrazono]-malononitrile (2)A well stirred solution of 4-aminoantipyrine (1.02 g, 5 mmol) in 2 N HCl (1.5 mL) was cooled in ice salt bath and diazotized with 1 N NaNO2 solution (0.35 g, 5 mmol; in 2 mL water). The mixture was then tested for complete diazotization using starch iodide paper which gives a weak blue test. If the mixture does not give the test, more sodium nitrite was added dropwise until a positive test is obtained and the color is stable for few minutes. If, on the other hand, strong test for nitrite is obtained, a few drops of a dilute solution of the base hydrochloride is added until the nitrite test is nearly negative. The above cold diazonium solution was added slowly to a well stirred solution to malononitrile (0.33 g, 5 mmol) in ethanol (20 mL) containing sodium acetate (0.43 g, 5.2 mmol) and the mixture was cooled in an ice salt bath. After the addition of the diazonium salt solution the reaction was tested for coupling reaction. A drop of the reaction mixture was placed on a filter paper and the colorless ring surrounding the spot dye was treated with a drop of an alkaline solution of a reactive coupler, such as sodium salt of 3-hydroxy-2-naphthanilide. If un-reacted diazonium salt is present, a dye is formed. The presence of un-reacted coupler can be determined in a similar manner using a diazonium salt solution to test the colorless ring. After the coupling reaction is complete, the reaction mixture was stirred for 50 minutes at room temperature. The crude product was filtered, dried and recrystallized from ethanol to give antipyrinylhydrazonomalononitrile (2) (93%), mp 140oC; yellowish orange crystals; 1H-NMR (400 MHz, DMSO-d6): δ, 2.26 (s, 3H, CH3), 3.25 (s, 3H, N-CH3), 7.35-7.56 (m, 5H, Ph), 12.1 (br., s, 1H, NH); MS: (m/z, %): 281 (M++1, 4.3), 280 (M+, 13.4), 188 (5.2), 91 (8.1), 56 (100.0).General procedure for the synthesis of 3-amino-2- (1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-[3-substituted]-1-yl-acrylonitriles 3-10A mixture of 2 (1.4 g, 5 mmol) and the appropriate secondary amine namely; piperidine (0.49 mL, 5 mmol), morpholine (0.43 mL, 5 mmol), N-methylglucamine (0.98 g, 5 mmol), pyrrolidine (0.41 mL, 5 mmol), diphenyl amine (0.85 g, 5 mmol), ethyl 2-(4-chlorophenylamino)acetate (1.07 g, 5 mmol), piperazine (0.43 g, 5 mmol) or 1-phenylpiperazine (0.81 g, 5 mmol) in ethanol (15 mL) was refluxed for 5 h. The reaction mixture was left to cool and the precipitated solid was filtered off, dried and recrystallized from EtOH/DMF (2:1) mixture to afford the corresponding acyclic enaminonitriles 3-10, respectively.3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-piperidin-1-yl-acrylonitrile (3)Yield (91%), mp 209oC; dark green crystals; IR (KBr) ύ (cm-1), 3392, 3334 (NH2), 3189 (NH), 2960 (C-H, stretching), 2171 (CN), 1639 (CO), 1448 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 1.58-1.69 (m, 6H, 3CH2, piperidine), 2.63 (s, 3H, CH3), 3.16 (s, 3H, N-CH3), 3.52-3.62 (m, 4H, 2CH2, piperidine), 7.13 (br., s, 2H, NH2), 7.31-7.52 ppm (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.2, 160.4, 160.1, 136.6, 136.5, 129.1, 129.0, 119.7, 119.5, 114.8, 113.2, 113.1, 113.0, 95.7, 46.8, 46.2, 46.1, 39.8, 25.9, 25.8, 25.7, 13.1 ppm. MS: (m/z, %): 367 (M+, 2.3), 366 (M+-1, 14.5), 338 (12.2), 280 (11.0), 215 (11.0), 189 (77.9), 152 (100.0), 86 (12.8), 63 (26.7). Anal. for C19H25N7O (367.45): Calcd. C, 62.10; H, 6.86; N, 26.68%; Found: C, 62.23; H, 6.91; N, 26.76%.3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-morpholin-4-yl-acrylonitrile (4)Yield (83%), mp 232oC; light brown crystals; IR (KBr) ύ (cm-1), 3385, 3337 (NH2), 3197 (NH), 2967 (C-H, stretching), 2186 (CN), 1637 (CO), 1470 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 2.22-2.25 (m, 4H, 2CH2, morpholine), 2.44 (s, 3H, CH3), 3.10 (s, 3H, N-CH3), 3.58-3.74 (m, 4H, 2CH2, morpholine), 7.24 (br., s, 2H, NH2), 7.36-7.51 ppm (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.2, 160.5, 160.3, 136.6, 136.5, 129.4, 129.1, 119.7, 119.5, 114.8, 113.3, 113.1, 113.0, 95.7, 67.2, 64.9, 47.1, 46.8, 39.8, 13.1 ppm. MS: (m/z, %): 368 (M+-1, 6.7), 367 (M+-2, 15.5), 275 (7.7), 214 (13.4), 188 (14.6), 108 (24.6), 96 (17.8), 56 (100.0); Anal. for C18H23N7O2 (369.42): Calcd. C, 58.52; H, 6.28; N, 26.54%; Found: C, 58.61; H, 6.33; N, 26.61%.3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-[methyl-(2,3,4,5,6-pentahydroxy-hexyl)-amino]-acrylonitrile (5)Yield (83%), mp 205oC; dark yellow crystals; IR (KBr) ύ (cm-1), 3451, 3436 (OH), 3358, 3301 (NH2), 2954 (C-H, stretching), 2186 (CN), 1648 (CO), 1459 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 2.47 (s, 3H, CH3), 3.16 (s, 3H, N-CH3), 3.35-3.41 (m, 5H, CH2-N-CH3), 3.86-3.93 (m, 2H, CH2O), 4.36-5.14 (br, m, 5H, 5OH), 7.33 (br., s, 2H, NH2), 7.35-7.53 ppm (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.3, 160.6, 160.1, 136.6, 136.5, 129.1, 129.3, 119.8, 119.5, 114.8, 113.5, 113.1, 113.1, 95.7, 72.9, 72.8, 71.6, 71.3, 70.8, 64.9, 51.6, 46.8, 39.8, 35.9, 13.2 ppm. MS: (m/z, %): 477 (M+, 100.0), 438 (97.0), 282 (78.8), 279 (48.5), 241 (93.9), 178 (69.7), 163 (57.6), 144 (63.6), 104 (45.5), 94 (15.2), 57 (30.3); Anal. for C21H31N7O6 (477.51): Calcd. C, 52.82; H, 6.54; N, 20.53%; Found: C, 52.91; H, 6.59; N, 20.72%.3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-pyrrolidin-1-yl-acrylonitrile (6)Yield (88%), mp 229oC; light brown sheets; IR (KBr) ύ (cm-1), 3367, 3272 (NH2), 3183 (NH), 2944, 2875 (C-H, aliphatic), 2173 (CN), 1641 (CO), 1467 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 1.92-2.09 (m, 4H, 2CH2, pyrrolidine), 2.44 (s, 3H, CH3), 3.10 (s, 3H, N-CH3), 3.50-3.69 (m, 4H, 2CH2, pyrrolidine), 6.73 (br., s, 2H, NH2), 7.31-7.51 ppm (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.3, 160.5, 160.1, 136.8, 136.5, 129.1, 129.0, 119.7, 119.6, 114.8, 113.4, 113.1, 113.0, 95.8, 49.5, 49.6, 26.2, 26.1, 26.0, 46.8, 39.8, 13.1 ppm. Anal. for C18H23N7O (353.42): Calcd.: C, 61.17; H, 6.56; N, 27.74%; Found: C, 61.26; H, 6.61; N, 27.83%.3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-diphenylamino-acrylonitrile (7)Yield (75%), mp 98oC; light black powder; IR (KBr) ύ (cm-1), 3352, 3271 (NH2), 2179 (CN), 1644 (CO), 1472 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 2.42 (s, 3H, CH3), 3.18 (s, 3H, N-CH3), 6.63-7.54 (m, 15H, Ar-H), 8.14 ppm (br., s, 2H, NH2); 13C-NMR (100 MHz, DMSO-d6): δ, 183.4, 160.4, 160.1, 140.8, 136.7, 136.5, 129.8, 129.7, 129.6, 129.2, 129.0, 119.7, 119.6, 119.2, 119.1, 118.7, 118.7, 118.6, 118.4, 114.8, 113.4, 113.1, 113.2, 95.7, 46.9, 39.8, 13.3 ppm. Anal. for C26H25N7O (451.52): Calcd.: C, 69.16; H, 5.58; N, 21.71%; Found: C, 69.27; H, 5.63; N, 21.79%.[1-Amino-2-cyano-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-vinyl]-(4-chloro-phenyl)-amino]-acetic acid ethyl ester (8)Yield (75%), mp 88-90oC; light black powder; IR (KBr) ύ (cm-1), 3358, 3266 (NH2), 2183 (CN), 1740 (C=O, ester), 1648 (CO), 1479 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 1.29 (t, 3H, CH2CH3, J= 7.2 Hz), 2.41 (s, 3H, CH3), 3.18 (s, 3H, N-CH3), 3.82 (s, 2H, CH2), 4.12 (q, 2H, CH2CH3, J= 7.2 Hz), 6.2 (br, s, 2H, NH2), 7.01-8.12 (m, 9H, Ar-H); 13C-NMR (100 MHz, DMSO-d6): δ, 183.2, 169.5, 160.5, 160.1, 142.3, 136.6, 136.5, 129.3, 129.1, 129.0, 122.8, 119.7, 119.6, 115.2, 115.3, 114.8, 113.3, 113.1, 113.0, 95.7, 62.1, 50.3, 46.8, 39.8, 14.8, 13.1. MS: (m/z, %): 495 (M+, 0.5), 447 (0.2), 214 (7.5), 212 (19.6), 141 (33.0), 139 (100.0), 56 (16.0); Anal. for C24H26ClN7O3 (495.96): Calcd.: C, 58.12; H, 5.28; N, 19.77%; Found: C, 58.21; H, 5.34; N, 19.81%.3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-piperazin-1-yl-acrylonitrile (9)Yield (72%), mp 89-90oC; dark red powder; IR (KBr) ύ (cm-1), 3450, 3379 (NH2), 3159 (NH), 2929 (C-H, stretching), 2174 (CN), 1639 (CO), 1494 (N=N); 13C-NMR (100 MHz, DMSO-d6): δ, 183.3, 160.4, 160.1, 136.7, 136.5, 129.1, 129.0, 119.7, 119.5, 114.8, 113.3, 113.1, 113.0, 95.7, 50.6, 50.5, 46.8, 46.9, 46.6, 39.8, 13.1. MS: (m/z, %): 368 (M+, 0.4), 343 (1.0), 228 (2.9), 201 (6.9), 189 (10.0), 160 (17.5), 135 (69.5), 73 (100.0), 65 (20.8); Anal. for C18H24N8O (368.44): Calcd.: C, 58.68; H, 6.57; N, 30.41%; Found: C, 58.63; H, 6.51; N, 30.38%.3-Amino-2-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylazo)-3-(4-phenyl-piperazin-1-yl)-acrylonitrile (10)Yield (86%), mp 230oC; yellow powder; IR (KBr) ύ (cm-1), 3390, 3334 (NH2), 2925, 2809 (C-H, aliphatic), 2173 (CN), 1610 (CO), 1490 (N=N); 1H-NMR (400 MHz, DMSO-d6): δ, 2.44 (s, 3H, CH3), 3.10 (s, 3H, N-CH3), 3.28-3.36 (m, 4H, 2CH2, piperazine), 3.72-3.82 (m, 4H, 2CH2, piperazine), 6.12 (br., s, 2H, NH2), 6.81-7.53 (m, 5H, Ph); 13C-NMR (100 MHz, DMSO-d6): δ, 183.2, 160.4, 160.1, 149.7, 136.6, 136.5, 130.2, 130.1, 129.1, 129.0, 119.7, 119.5, 118.4, 114.8, 114.4, 114.3, 113.2, 113.1, 113.0, 95.7, 50.7, 50.6, 47.3, 46.8, 39.8, 13.1. MS: (m/z, %): 444 (M+, 5.0), 375 (0.4), 228 (46.6), 214 (65.3), 188 (82.4), 162 (59.7), 132 (94.7), 120 (100.0), 99 (67.3), 88 (42.7), 73 (81.9), 66 (24.3); Anal. for C24H28N8O (444.53): Calcd.: C, 64.84; H, 6.35; N, 25.21%; Found: C, 64.92; H, 6.39; N, 25.27%.
4.2. Dyeing Procedures
4.2.1. Preparation of Dye Dispersion
- The required amount of the dye (2% shade) was dissolved in suitable solvent (DMF) and added drop wise with stirring to a solution of Dekol-N (2 g/dm3), an anionic dispersing agent of BASF, then the dye was precipitated in a fine dispersion ready for use in dyeing.
4.2.2. Dyeing of Polyester at 130oC under Pressure Using Fescaben as a Carrier
- The dye bath (1:20 liquor ratio) containing 5 g/ dm3 5 g dm-3 Levegal PT (Bayer) as a carrier, 4% ammonium sulphate and acetic acid at pH= 5.5, was brought to 60oC. The polyester fabric was entered at this degree and run for 15 minutes. 2% Dye in the fine dispersion was added, temperature was raise to the boil within 45 minutes, dyeing was continued at the boil for about 1 hour, then dyed material was rinsed and soaped with 2% nonionic detergent to improve rubbing and wet fastness.
4.2.3. Assessment of Colour Fastness (Tables 2)
- Fastness to washing, perspiration, light and sublimation was tested according to the reported methods.i. Fastness to washingA specimen of dyed polyester fabric was stitched between two pieces of un-dyed cotton fabric, all of equal diameters and then washed at 50oC for 30 minutes. The staining on the un-dyed adjacent fabric was assessed according to the following grey scale: 1-poor, 2-fair, 3-moderate, and 4- good, 5-excellent.ii. Fastness to perspirationThe samples were prepared by stitching pieces of dyed polyester fabric between two pieces of un-dyed cotton fabric, all of equal diameters and then immersed in the acid medium for 30 minutes. The staining on the un-dyed adjacent fabric was assessed according to the following grey scale: 1-poor, 2-fair, 3-moderate, and 4-good, 5-excellent. The acid solution (pH= 3.5) contain sodium chloride 10 g/l, lactic acid 1 g/dm3, disodium orthophosphate 1 g/dm3 and histidine monohydrochloride 0.25 g/dm3.iii. Fastness to rubbingThe dyed polyester fabric was placed on the base of Crocketeer, so that it rests flat on the abrasive cloth with its long dimension in the direction of rubbing. A square of white testing cloth was allowed to slide on the tested fabric back and forth twenty times by making ten complete turns of the crank. For wet rubbing test, the testing square was thoroughly wet in distilled water. The rest of the procedure is the same as the dry test. The staining on the white testing closed was assessed according to grey scale: 1-poor, 2-fair, 3-moderate, and 4-good, 5-excellent.iv. Fastness to sublimationSublimation fastness was measured with an iron tester (Yasuda no. 138). The samples were prepared by stitching pieces of dyed polyester fabric between two pieces of un-dyed polyester, all of equal diameters and then treated at 180oC and 210oC for 1 min. Any staining on the un-dyed adjacent fabric or change in tone was assessed according to the following grey scale: 1-poor, 2-fair, 3-moderate, and 4-good, 5-excellent.v. Fastness to lightLight fastness was determined by exposing the dyed polyester on a Xenotest 150 (Original Hanau, chamber temperature 25-30oC, black panel temperature 60oC, relative humidity 50-60%, and dark glass (UV) filter system) for 40 hours. The changes in colour were assessed according to the following blue scale: 1-poor, 3-moderate, 5-good, and 8-very good.
4.2.4. Colour Assessment
- Tables (1) report the colour parameters of the dye fabrics assessed by tristimulus colorimetry. The colour parameters of the dyed fabrics were determined on a spectro multichannel photo detector (model MCPD1110A), equipped with a D65 source and barium sulphate as a standard blank. The values of the chromaticity coordinates, luminance factor and the position of the color in the CIELAB color solid are reported.In this study, the dyeing performance of the prepared dyes 2-10 on polyester fibres has been evaluated. The results are listed in Table 2. Generally, the fastness properties of dyes 2-10 on polyester fibres were studied (Table 2) and it was observed that: (a) Fastness to washing on polyester fibres is generally acceptable (3-5), according to the International Geometric Grey Scale. (b) These dyeing showed good stability to acid perspiration (rating 4-5). (c) The light fastness ranges are 7-8 on polyester fibres. (d) All of the dyes have acceptable fastness to rubbing (4-6) for wet and dry fibres. This may be attributed to good penetration.
References
| [1] | A. A. Fadda, S. Bondock, R. Rabie, H. A. Etman, Eur. J. Med. Chem. 43, 2122 (2008). |
| [2] | E. Abdel-Latif, , 125 (2006). |
| [3] | A. A. Fadda, S. Bondock, R. Rabie, Monatsheft Chem. 139, 153 (2008). |
| [4] | D. G. Carciunescu, R. An, Acad. Farm. 43, 265 (1977). |
| [5] | J. Hosler, C. Tschanz, C. E. Hignite, D. L. J. Azarnoff, Invest. Dcrmatol. 74, 51 (1986). |
| [6] | P. J. Meffin, R. L. Williams, T. F. Blaschke, M. J. Rowland, Pharm. Sci. 66, 135 (1977). |
| [7] | E. V. Schmidt, T. P. Prishchep, N. A. Chernova, Izv Tomsk Politekbn Inst. 156 (1975). |
| [8] | S. C. Jain, J. Sinha, S. Bhagat, W. Errington, C. E. Olsen, Synth. Commun. 33, 563 (2003). |
| [9] | F. V. Cechinel, R. Correa, Z. Vaz, J. B. Calixto, R. J. Nunes, T. R. Pinheiro, A. D. Andricopulo, R. A. Yunes, Il Farmaco 53, 55 (1998). |
| [10] | S. M. Sondhi, V. K. Sharma, R. P. Verma, N. Singhal, R. Shukla, R. Raghubir, M. P. Dubey, Synthesis 878 (1999). |
| [11] | A. P. Mishra, J. Indian Chem. Soc. 76, 35 (1999). |
| [12] | N. Raman, A. Kulandaisamy, K. Jeyasubramanian, Syn. React. Inorg. Met. 32, 1583 (2002). |
| [13] | N. Raman, A. Kulandaisamy, K. Jeyasubramanian, Syn. React. Inorg. Met. 34, 17 (2004). |
| [14] | S. M. Sondhi, N. Singhal, R. P. Verma, S. K. Arora, S. G. Dastidar, Indian J. Chem. 40(B), 113 (2001). |
| [15] | P. G. Sushama, M. Alaudeen, Asian J. Chem. 15(1), 366-372 (2003). |
| [16] | A. Pohlmann, W. W. Stamm, H. Kusakabe, M.-R. Kula, Analytica Chim. Acta 235(2), 329-35 (1990). |
| [17] | A. Maquestiau and J.-J. Vanden Eynde, Bull. des Soc. Chim. Belges 95(8), 641-8 (1986). |
| [18] | G. E. H. Elgemeie, F. A. M. Abd El Aal, Heterocycles 24(2), 349-53 (1986). |
| [19] | M. H. Elnagdi, H. A. Elfahham, M. R. H. Elmoghayar, K. U. Sadek, G. E. H. Elgemeie, J. Chem. Soc., Perkin Trans. 1, (4), 989-91 (1982). |
| [20] | A. A. Fadda, H. A. Etman, F. A. Amer, M. Barghout, Kh. S. Mohamed, J. Chem. Tech. Biotechnol. 62, 165-169 (1995). |
| [21] | A. A. Fadda, H. A. Etman, S. E. El-Desoky, S. Bondk, J. Chem. Tech. Biotechnol. 64, 393-397 (1995). |
| [22] | A. A. Fadda, M. M. Ali, A. S. El-Ahl, A. Fouda, Indian J. Fibre & Tex. Res. 18, 151-155 (1993). |
| [23] | A. A. Fadda, H. A. Etman, M. M. Ali, A. Fouda, Indian J. Fibre & Tex. Res. 20, 34-42 (1995). |
| [24] | A. A. Fadda, H. A. Etman, F. A. Amer, M. Barghout, Kh. M. Samir, J. Chem. Tech. Biotechnol. 62, 170-177 (1995). |
| [25] | A. M. Khalil, M. A. Berghot, M. A. Gouda, Monatsh. Chem., 140 (11), 1371-1379 (2009). |
| [26] | A. M. Khalil, M. A. Berghot, M. A. Gouda, S. A. El Bialy Monatsh. Chem. 141, 1353-1360 (2010). |
| [27] | E. Abdel-Latif, F. A. Amer, Monatsh. Chem., 139, 561-567 (2008). |
| [28] | V. Kryštof, P. Cankař, I. Fryšová, J. Slouka, G. Kontopidis, P. Džubák, M. Hajdúch, J. Srovnal, W. F. de AzevedoJr., M. Orság, M. Paprskářová, J. Rol ík, A. Látr, P. M. Fischer, M. Strnad, J. Med. Chem. 49, 6500-6509 (2006). ík, A. Látr, P. M. Fischer, M. Strnad, J. Med. Chem. 49, 6500-6509 (2006). |
| [29] | A. A. Fadda, S. S. Elmorsy, A. M. El-Sayed, A. M. Khalil, S. A. Elagazy Indian J. Fibre Text. Res. 15, 190 (1990). |
| [30] | Society of Dyes and Colorists, Standard methods for the determination of color fastness of textiles and leather, 5th edn Society of Dyes and Colorists, Bradfo (1990). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML