Osman M. O. Habib , Hussein M. Hassan, Ahmed El-Meka
Department of Chemistry, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
Correspondence to: Hussein M. Hassan, Ahmed El-Meka , Department of Chemistry, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
2-(4-Toluenesulphonyloxy phenyl)-3,1-benzoxazine-4-one 2 was prepared and reacted with some nitrogen nucleophiles, e.g., ammonia, o-phenylendiamine, some heterocyclic amines, hydrazine hydrate and hydroxylamine hydrochloride and sulphur nucleophile, e.g., phosphorous pentasulphide. Structures of the newly synthesized compounds were established by elemental analysis and spectral data. All new prepared compounds were subjected to antimicrobial activity evaluation where compounds, 8, 9, 10 and 18 exhibited good activities against Bacillus Thuringenesis and 5, 10, 11, 14, 15, 17 and 18 exhibited good activities against Klebseilla Pneumonia. On the other hand, the results for antifungal activities revealed that, compounds 5, 6, 7, 16 and 17 exhibited good activities against Trichoderma Herzianum and Trichoderma Virdi.
Keywords:
Quinazolinone, Tetrazole, Thiazolidinone, Imidazole, Antibacterial
1. Introduction
Substituted benzoxazinone and quinazolinone derivatives have become of great importance due to their wide range of biological activity. Previous studies have reported that, they exhibit antitubercular, antihypertensive, anticancer, anti- HIV, antiviral, anti-inflammatory, and antifungal activities[1,2]. Besides, they were used as analgesics, inhibitors for cathepsin G, Human leukocyte elastase, dual selective serotonin reuptake, and potent inactivators of CIr serine protease[3]. On the other hand, it has been stated that compounds containing aromatic sulfonate or sulfonamide moieties possess high acaricidal as well as insecticidal activity[4].
2. Results and Discussions
As a part of our interest on the synthesis of biologically active molecules, the present investigation aims to synthesize a series of products bearing both aryl sulfonate, benzoxazinone and quinazolone moieties in the same molecule hoping that, these new products might show high biocide activity. Thus, 2-(4-toluene sulphonyloxy phenyl) -3,1- benzoxazine-4-one 2 was prepared by treatment of one mole of anthranilic acid with two moles of the acid chloride 1 in the presence of dry pyridine (Scheme 1)[5]. Fusion of compound 2 with o-phenylenediamine in the presence of freshly fused sodium acetate at 100℃ and 180℃gives different products. When fusion was carried out at 100℃, 4–(3–(2–aminophenyl)–4–oxo–3,4–dihydro quinazolin–2–yl) phenyl–4-methyl benzene sulfonate 4 was obtained. On the other hand, fusion of 2 at 180℃ leads to the formation of 4–(4–benzo[4,5]imidazo[1,2–c] quinazolin –6–yl) phenyl– 4–methylbenzene sulfonate 3. Moreover, reaction of benzoxazinone 2 with o-phenylenediamine in absolute ethanol under reflux afforded 4–[2–[(N-2- aminophenyl) benzoyl] carbamoyl]phenyl–4'–methyl benzenesulfonate 5. Furthermore, fusion of 2 with ammonium acetate at 160-170°C afforded 4–(4–oxo–3,4–dihydroquinazolin –2–yl) phenyl–4'–methylbenzenesulfonate 6 (Scheme 2). The infra-red spectrum of 6 revealed stretching frequencies at 3336, 3173, cm-1 characteristic for the –OH and -NH, groups, respectively. This illustrates that quinazolone derivative 6 exists in a lactam- lactim tautomeric equilibrium.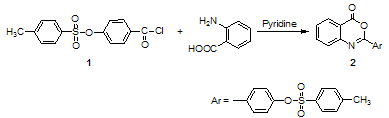 | Scheme 1. Synthesis of 4-(4-oxo-4H-benzo[d][1,3]oxazin-2-yl)phenyl 4-methylbenzene-sulfonate (2) |
On the other hand, compound 2 reacted with some heterocyclic amines, e.g., 4-aminoantipyrine, 2-aminopyridine and 3-aminopyridine afforded 7, 8 and 9 respectively. Moreover, benzoxazinone 2 reacts with phosphorous pentasulfide in dry xylene under reflux affording 4-(4-thioxo- 4H-3,1-benzothiazin-2-yl)phenyl-4-methylbenzenesulfonate 10 (Scheme 3). With the aim of expanding the synthetic potential of the quinazolones formed, we also studied the reaction of benzoxazinone 2 with both hydroxylamine hydrochloride and hydrazine hydrate. Thus, the reaction of 2 with hydroxylamine hydrochloride in ethanol in the presence of sodium acetate gives 4–(3-hydroxy–4–oxo–3,4– dihydroquinazolin–2–yl)phenyl–4–methyl benzene sulfonate 11. On the other hand, reaction of 2 with hydrazine hydrate in ethanol revealed 4–(3-amino–4–oxo–3,4– dihydriquinazolin–2–yl)phenyl-4–methyl benzene sulfonate 12 (Scheme 3).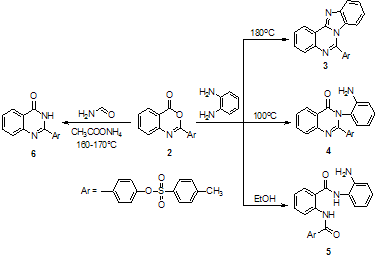 | Scheme 2. Reaction of benzoxazinone 2 with o-phenylenediamine and formamide |
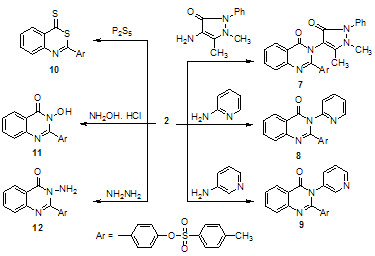 | Scheme 3. Condensation of benzoxazinone 2 with different amines and phosphorus pentasulphide |
Condensation of compound 12 with benzaldehyde in boiling ethanol containing few drops of piperidine afforded the corresponding 4–(3–(benzylideneamino)–4–oxo–3,4– dihydroquinazolin–2–yl)phenyl–4–methylbenzenesulfonate 13. It was thus of interest in the present work to prepare some new products containing in their skeleton both the azetidinone and thiazolidinone moieties linked with quinazolone derivatives. Thus reaction of 13 with both chloroacetyl chloride and thioglycolic acid gave 14 and 15, respectively. Moreover, acetylation and benzoylation of 12 with acetylchloride and/or benzoylchloride afforded 4–(3,3–diacet- amido–4–oxo-3,4–dihydroquinazolin–2–yl)phenyl–4–methylbenzenesulfonate 16 and 4-(4-oxo-3,3-(dibenzamido)-3, 4-dihydroquinazolin-2-yl)phenyl-4-methyl benzene sulfonate 17, respectively. Furthermore, when 3- aminoquinazolinone 12 condensed with phthalic anhydride by fusion in an oil bath at 160℃ gives4–(3–(1,3–dioxoisoindolin-2–yl)–4–oxo–3, 4–dihydroquinazolin–2–yl) phenyl–4– methyl benzene sulfonate 18 (Scheme 4).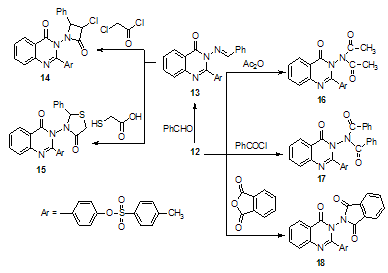 | Scheme 4. Reactions of 3-aminoquinazolone derivative 12 |
3. Antimicrobial Activity
Most of the compounds showed very good antibacterial and antifungal activities and were almost competitive with the standard drugs. The results are summarized in Table I, II.The results for antibacterial activities depicted in Table I revealed that, compounds 3, 5, 7, 8, 9, 10, 11, 17 and 18 exhibited good activities against the reference chemotherapeutics due to the presence of antipyrine, pyridine and phthalimide moieties which play an important as antibacterial and antifungal, while 8, 9, 10 and 18 exhibited good activities against Bacillus Thuringenesis and compounds 5, 10, 11, 14, 15, 17 and 18 exhibited good activities against Klebseilla Pneumonia. On the other hand, the results for antifungal activities depicted in Table II revealed that, compounds 5, 6, 7, 16 and 17 exhibited good activities against Trichoderma Herzianum and Trichoderma Virdi. A comparison of antibacterial and antifungal activities of compounds with their structures revealed that, the compounds that bearing aryl sulfonate, benzoxazinone and quinazolone moieties in the same molecule caused significant activity against Bacillus Thuringenesis, Klebseilla Pneumonia, Trichoderma Herzianum and Trichoderma Virdi.
4. Experimental
All melting points are recorded on Gallenkamp electric melting point apparatus. The IR spectra υ cm-1 (KBr) were recorded on Perkin ElmerInfrared Spectrophotometer Model 157, Grating. The 1H NMR spectra were obtained on a Varian Spectrophotometer at 500 MHz using TMS as an internal reference and DMSO-d6 as solvent. The mass spectra (EI) were recorded on 70 eV with Kratos MS equipment and/or a Varian MAT 311 A Spectrometer. Elemental analyses were carried out at the Micro Analytical Center of Cairo Univ., Giza, Egypt.| Table 1. Diameter of inhibited zones (I. Z. D.) in millimeters as a criterion of antibacterial activity of the synthesized compounds |
| | Comp. No. | Bacteria | Comp No. | Bacteria | | B. Thuringenesis | K. Pneumonia | B. Thuringenesis | K. Pneumonia | | I. Z.D. mm | I. Z.D. mm | I. Z.D. mm | I. Z.D. mm | | 2 | 27 | 33 | 12 | 27 | 35 | | 3 | 31 | 36 | 13 | 30 | 26 | | 4 | 27 | 30 | 14 | 29 | 45 | | 5 | 35 | 44 | 15 | 29 | 45 | | 6 | 29 | 33 | 16 | 32 | 39 | | 7 | 34 | 37 | 17 | 35 | 45 | | 8 | 39 | 38 | 18 | 39 | 50 | | 9 | 38 | 32 | Flummox | 24 | 29 | | 10 | 46 | 49 | Ampicillin | 29 | 34 | | 11 | 29 | 44 | Chloramphenicol | 19 | 28 |
|
|
| Table 2. Diameter of inhibited zones (I. Z. D.) in millimeters as a criterion of antifungal activity of the synthesized compounds at a concentration of 10 mg/ml |
| | Comp No. | Fungi | Comp No. | Fungi | | T. Herzianum | T. Virdi | T. Herzianum | T. Virdi | | I. Z.D. mm | I. Z.D. mm | I. Z.D. mm | I. Z.D. mm | | 2 | 35 | 39 | 12 | 31 | 48 | | 3 | 41 | 31 | 13 | 35 | 26 | | 4 | 37 | 32 | 14 | 33 | 39 | | 5 | 44 | 36 | 15 | 31 | 36 | | 6 | 49 | 40 | 16 | 48 | 49 | | 7 | 46 | 44 | 17 | 46 | 41 | | 8 | 40 | 48 | 18 | 40 | 46 | | 9 | 41 | 45 | Flummox | 35 | 41 | | 10 | 38 | 46 | Ampicillin | 41 | 36 | | 11 | 32 | 49 | Chloramphenicol | 32 | 31 |
|
|
Synthesis of 2-(4-toluenesulphonyloxyphenyl)-3,1- Benzoxazine- 4-one (2). To a solution of anthranilic acid (1.371 g, 0.01 mole) in dry pyridine (30 mL), the acid chloride 1 (6.21 g, 0.02 mole) was added portion wise with stirring at room temperature. The reaction mixture was poured onto cold water (100 mL) and the precipitated solid was filtered off, washed with cold water, dried and recrystallized from ethanol to give benzoxazinone derivative 2.Yellow crystals; Yield 50%; m.p. 140-141 oC; Anal. Calcd. for C21H15NO5S: C, 64.11; H, 3.84; N, 3.56. Found: C, 64.12; H, 3.77; N, 3.51; IR (KBr, cm-1): 1766 (CO of lactone), 1627 (C=N), 1370 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 8.21 (d, 1H, Ar-H), 8.09 (t, 1H, Ar-H), 7.79 (t, 1H, Ar-H), 7.66 (d, 1H, Ar-H), 7.96 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.40 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm):159(C=O), 156.3(C=N), 21.3(CH3), 128.2, 124.1, 135.2, 126.2, 146.1, 130.6, 116.0, 126.7, 130.3, 132.4, 138.2, 122.4, 152.5 (aromatic); MS (m/z, (relative abundance, %)): 393 (M+, 29.24), 374 (22.5), 261 (17), 177 (33.5), 105 (35.3), 91 (100). Synthesis of 4-(4–benzo[4,5]imidazo[1,2-c] quinazoline-6-yl)phenyl-4-methylbenzenesulfonate (3) and 4- (3–(2-aminophenyl)–4–oxo–3,4–dihydroquinazolin–2–yl) phenyl-4-methylbenzenesulfonate (4). A mixture of benzoxazinone 2 (1.18 g, 0.003 mole), o-phenylenediamine (0.32 g, 0.003 mole) and freshly fused sodium acetate (0.2 g) was fused at 180℃ and/or at 100℃ for 3 h. In each case, the reaction mixture was cooled, washed with dil. HCl. The separated solid product was dried and recrystallized from a mixture of ether-ethanol and methanol to give 3 and 4, respectively. Compound 3Brown crystals; Yield 61%; m.p. 291-293℃; Anal. Calcd. for C27H19N3O3S: C, 69.66; H, 4.11; N, 9.03. Found: C, 69.33; H, 4.00; N, 9.00; IR (KBr, cm-1): 1600 (C=N), 1360 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 7.84 (d, 1H, Ar-H), 7.58 (t, 1H, Ar-H), 7.83 (t, 1H, Ar-H), 8.16 (d, 1H, Ar-H), 7.91 (d, 2H, Ar-H), 7.01 (d, 2H, Ar-H), 7.59 (d, 2H, Ar-H), 7.22 (m, 2H, Ar-H), 7.56 (d, 1H, Ar-H), 7.74 (d, 2H, Ar-H), 7.40 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ/ ppm): 142.9, 161(2C=N), 21.3(CH3), 122.8, 127.7, 132.2, 128.5, 149.3, 118.7, 144.0, 120.1, 123.0, 112.1, 130.7, 116.4, 135.8, 150.2, 126.7, 130.3, 132.4, 138.2 (aromatic); MS (m/z, (relative abundance, %)): 467 (M++2, 0.96), 354 (11.4), 255 (13.4), 167 (55.8), 105 (15.8), 91 (100). Compound 4Brown crystals; Yield 55%; m.p. 170-172℃, Anal. Calcd. for C27H21N3O4S: C, 67.07; H, 4.38; N, 8.69. Found: C, 67.00; H, 4.28; N, 8.34; IR (KBr, cm-1): 3332-3425 (NH2), 1665 (CO, amidic), 1600 (C=N), 1360 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 3.3 (s, 2H, NH2), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 6.96 (d, 1H, Ar-H), 7.40 (t, 1H, Ar-H), 7.56 (d, 1H, Ar-H), 6.79 (t, 1H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.45 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 156(C=N), 21.3(CH3), 160(C=O), 126.6, 127.3, 133.4, 126.7, 148.7, 118.7, 120.8, 126.8, 134.1, 114.5, 125.1, 118.9, 114.8, 131.8, 116.0, 121.3, 151.6, 126.7, 130.3, 132.4, 138.2 (aromatic); MS (m/z, (relative abundance, %)): 483 (M+, 7.19), 389 (31.5), 298 (3.3), 177 (45.3), 165 (3.3), 91 (100).Synthesis of 4–[2–[(N-2-aminophenyl) benzoyl]carbamo yl]phenyl–4'–methylbenzenesulfonate (5). A mixture of benzoxazinone 2 (1.18 g, 0.003 mole) and o-phenylenediamine (0.32gm, 0.003 mole) in ethanol (20 mL) was refluxed for 8 hours. The solid product that separated on cooling was filtered off and recrystallized from ethanol to give 5.White crystals; Yield 33%; m.p. 225-227℃; Anal. Calcd. for C27H23N3O5S: C, 64.66; H, 4.62; N, 8.38. Found: C, 64.42; H, 4.54; N, 8.45; IR (KBr, cm-1): 3332-3425 (NH2), 3240 (-NH), 1665 (CONH), 1375 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 8.4 (s, 2H, NH2), 8.6 (s, 2H, -2NHCO), 7.87 (d, 1H, Ar-H), 7.37 (t, 1H, Ar-H), 7.68 (t, 1H, Ar-H), 8.37 (d, 1H, Ar-H), 6.98 (d, 1H, Ar-H), 7.40 (t, 1H, Ar-H), 6.79 (t, 1H, Ar-H), 8 (t, 1H, Ar-H), 7.86 (d, 2H, Ar-H), 7.13 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.40 (d, 2H, Ar-H); 13C-NMR(400 MHz,DMSO-d6, δ/ppm): 164.5, 167.7 (2CONH), 21.3(CH3), 127.6, 124.4, 132.4, 119.4, 138.9, 123.2, 122.8, 149.5, 114.5, 125.1, 118.9, 125.5, 128.8, 116.0, 126.8, 153.6, 126.6, 130.3, 132.4, 138.1 (aromatic);MS (m/z, (relative abundance, %)): 501 (M+, 4.13), 479 (1.8), 382 (22.5), 198 (12.4), 115 (100), 86 (14.9).Synthesis of 4-(4-oxo-3,4-dihydroquinazolin-2-yl)phenyl -4-methyl benzenesulfonate (6). A mixture of benzoxazinone 2 (3.93 g, 0.01 mole) and ammonium acetate (2.68 g, 0.01 mole) was fused in an oil bath at 160-170°C for 3 h. The reaction mixture was left to cool, washed with water several times, filtered off, dried and recrystallized from ethanol to give 6.Grey crystals; Yield 55%; m.p. 215-217℃; Anal. Calcd. for C21H16N2O4S: C, 64.27; H, 4.11; N, 7.14. Found: C, 64.15; H, 4.06; N, 7.06; IR (KBr, cm-1): 3336 (OH), 3173 (-NH), 1661 (CONH), 1604 (CN), 1377 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 3.5 (s, 1H, NH), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.75 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 161.0(C=O), 152.3(C=N), 21.3(CH3), 126.6, 127.4, 133.4, 126.7, 148.9, 120.8, 131.8, 116.0, 125.0, 151.6, 126.9, 130.4, 132.4, 138.2 (aromatic);MS (m/z, (relative abundance, %)): 392 (M+, 67.35), 356 (4.6), 287 (55.3), 178 (13.4), 105 (100), 85 (34.5).Synthesis of 4-(3-(1,5-dimethyl-3-oxo-2-phenyl -2,3- dihyhro-1H-pyrazol-4-yl)-4-oxo-3,4-dihydroquinazolin-2 -yl) phenyl-4-methyl benzene sulfonate (7). A mixture of 2 (0.79 g, 0.002 mole) and 4-aminoantipyrin (0.41 g, 0.002 mole) in glacial acetic acid (20 mL) was refluxed for 6 hours then cooled. The reaction mixture was poured onto ice water; the precipitated product was filtered off and recrystallized from acetic acid to give product 7.White crystals; Yield 83%; m.p. 195-197℃; Anal. Calcd. for C32H26N4O5S: C, 66.42; H, 4.53; N, 9.68. Found: C, 66.33; H, 4.48; N, 9.63; IR (KBr, cm-1): 1665 (CO, amidic), 1613 (CN), 1380 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 3.6 (s, 3H, CH3), 2.7 (s, 3H, CH3), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.35 (d, 2H, Ar-H), 7.37 (t, 2H, Ar-H), 6.09 (t, 1H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 160.7, 165.2(2C=O), 155.9(C=N), 21.3, 25.6(2CH3), 103.4, 133.7(C=C), 35.6(N-CH3),126.6, 127.4, 133.4, 126.7, 148.9, 120.8, 131.8, 116.0, 125.0, 151.6, 126.9, 130.4, 132.4, 138.2, 123.2, 129.2, 133.9, 122.8 (aromatic); MS (m/z, (relative abundance, %)): 578 (M+, 4.00), 445 (16.5), 397 (44.2), 258 (35.3), 175 (100), 117 (25.7).Synthesis of 4-(4-oxo-3-(pyridin-2-yl)-3,4 dihydroquinazolin-2-yl)phenyl 4-methyl benzenesulfonate (8). A mixture of benzoxazinone 2 (3.93 g, 0.01 mole) and 2-aminopyridine (0.94 g, 0.01 mole) was fused in an oil bath at 150-155℃ in presence of anhydrous ZnCl2 (1 g) for 4 h. The reaction mixture was triturated with ice/HCl. The formed solid product was filtered off, washed with water several times, dried and recrystallized from methanol to give 8.White crystals; Yield 68%; m.p. 88-90℃; Anal. Calcd. for C26H19N3O4S: C, 66.51; H, 4.08; N, 8.95. Found: C, 66.34; H, 4.06; N, 8.68; IR (KBr, cm-1): 1685 (CO, amidic), 1597 (CN), 1374 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 6.70 (d, 1H, Ar-H), 7.55 (t, 1H, Ar-H), 6.62 (t, 1H, Ar-H), 8.07 (d, 1H, Ar-H) 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ /ppm): 160.7(C=O), 156.2(C=N), 21.3(CH3), 126.6, 127.4, 133.4, 126.7, 148.9, 120.8, 131.8, 116.0, 125.0, 151.6, 126.9, 130.4, 132.4, 138.2, 123.9, 147.6, 138.3, 117.9, 148.1 (aromatic); MS (m/z, (relative abundance, %)): 469 (M+, 3.24), 433 (6.7), 356 (55.7), 248 (25.5), 105 (100), 86 (33.8).Synthesis of 4-(4-oxo-3-(pyridine-3-yl)-3,4–dihydroquin- azolin-2-yl) phenyl 4-methyl benzenesulfonate (9). A mixture of benzoxazinone 2 (3.93 g, 0.01 mole) and 3-aminopyridine (0.94 g, 0.01 mole) was fused in an oil bath at 150-155℃ in presence of anhydrous ZnCl2 (1 g) for 4 h. The reaction mixture was triturated with ice/HCl. The formed solid product was filtered off, washed with water several times, dried and recrystallized from methanol to give 9.White crystals; Yield 79%; m.p. 112-114℃; Anal. Calcd. for C26H19N3O4S: C, 66.51; H, 4.08; N, 8.95. Found: C, 66.22; H, 3.96; N, 8.87; IR (KBr, cm-1): 1685 (CO, amidic), 1597 (CN), 1374 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 8.03 (d, 1H, Ar-H), 7.63 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.27 (d, 1H, Ar-H), 7.36 (t, 1H, Ar-H), 8.09 (d, 1H, Ar-H), 8.02 (s, 1H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 160.7(C=O), 156.2, 138.8(2C=N), 21.3(CH3), 126.6, 127.4, 133.4, 126.7, 148.9, 120.8, 131.8, 116.0, 125.0, 151.6, 126.9, 130.4, 132.4, 138.2, 122.8, 145.1, 137.5, 124.7 (aromatic); MS (m/z, (relative abundance, %)): 469 (M+, 5.88), 446 (13.3), 366 (37.6), 237 (38.5), 165 (100), 112 (43.7). Synthesis of 4-(4-thioxo-4H-3,1-benzothiazin-2-yl)phenyl-4-methyl benzenesulfonate(10). A mixture of benzoxazinone 2 (3.93g, 0.01 mole) and phosphorous pentasulphide (8.9 g, 0.02 mole) in dry xylene (40 mL) was refluxed for 8 h. The reaction mixture was filtered off while hot, concentrated and the solid that separated on cooling was washed with petroleum ether (b.p. 80-100), then recrystallized from ethanol to give 10.Yellow crystals; Yield 38%; m.p. 135-137℃; Anal. Calcd. for C21H15NO3S3: C, 59.27; H, 3.55; N, 3.29. Found: C, 59.18; H, 3.50; N, 3.24; IR (KBr, cm-1): 1323 (CS), 1595 (C=N), 1364 (SO3). 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 7.34 (d, 1H, Ar-H), 7.45 (m, 3H, Ar-H), 7.66 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 218(C=S), 165.0(C=N), 21.3(CH3), 131.0, 126.6, 132.0, 126.0, 153.3, 135.4, 130.6, 116.0, 128.8, 152.5, 126.7, 130.3, 132.4, 138.2 (aromatic); MS (m/z, (relative abundance, %)): 425 (M+, 24.46), 385 (24.5), 256 (7.8), 227 (42.6), 187 (100), 91 (33.5).Synthesis of4-(3-hydroxy-4-oxo-3,4-dihydroquinazo-lin-2-yl)phenyl -4-methylbenzenesulfonate (11). To a solution of benzoxazinone 2 (2.35 g, 0.006 mole) in ethanol (30 mL), hydroxylamine hydrochloride (0.417 g, 0.006 mole) and sodium acetate (0.49 g, 0.006 mole) dissolved in the least amount of water was added. The reaction mixture was refluxed for 8 h, cooled and then concentrated. The solid product that separated on cooling was filtered off and recrystallized from ethanol to give 11.Yellow crystals; Yield 62%; m.p. 170-172℃; Anal. Calcd. for C21H16N2O5S: C, 61.76; H, 3.95; N, 6.86. Found: C, 61.66; H, 4.00; N, 6.84; IR (KBr, cm-1): 3220 (OH), 1665 (CON), 1613 (C=N), 1380 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 3.7 (s, 1H, OH), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 155(C=O), 156.2(C=N), 21.3(CH3), 126.6, 127.3, 133.4, 126.7, 148.7, 120.8, 131.8, 116.5, 121.2, 151.6, 126.7, 130.3, 132.4, 138.2 (aromatic); MS (m/z, (relative abundance, %)): 408 (M+, 5.70), 356 (4.15), 243 (23.4), 167 (53.2), 105 (100), 86 (24.7).Synthesis of 4-(3-amino-4-oxo-3,4-dihydroquinazo lin-2- yl)phenyl -4 -methylbenzenesulfonate (12). A solution of benzoxazinone 2 (3.93 g, 0.01 mole) and hydrazine hydrate (1.0 g, 0.02 mole) in absolute ethanol (30 mL) was refluxed for 6 hours. The solid product that separated on cooling was filtered off, dried and recrystallized from ethanol to afford the quinazolinone derivative 12.Yellow crystals; Yield 60%; m.p. 156-158℃; Anal. Calcd. for C21H17N3O4S: C, 61.90; H, 4.21; N, 10.31. Found: C, 61.80; H, 4.20; N, 10.22; IR (KBr, cm-1): 3332-3425 (NH2), 1653 (CON), 1597 (C=N), 1360 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 4.9 (s, 2H, NH2), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 155(C=O), 156.2(C=N), 21.3(CH3), 126.6, 127.3, 133.4, 126.7, 148.7, 120.8, 131.8, 116.5, 121.2, 151.6, 126.7, 130.3, 132.4, 138.2 (aromatic); MS (m/z, (relative abundance, %)): 407 (M+, 100), 386 (15.65), 295 (3.8), 158 (11.2), 115 (10.1), 85 (34.3).Synthesis of 4-(3–benzylideneami-no)-4–oxo–3,4– dihydroquinazolin–2–yl) phenyl-4–methyl benzenesulfonate (13). To a solution of 12 (4.07 g, 0.01 mole) in absolute ethanol (30 mL) containing few drops of piperidine, benzaldehyde (1.06 g, 0.01 mole) was added. The reaction mixture was refluxed for 5 h, concentrated and left to cool. The precipitated product was filtered off and recrystallized from ethanol to give 13.Yellow crystals; Yield 62%; m.p. 180-182℃; Anal. Calcd. for C28H21N3O4S : C, 67.88; H, 4.24; N, 14.85. Found: C, 67.56; H, 4.18; N, 14.82; IR (KBr, cm-1): 1678 (CON), 1596 (C=N), 1360 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 9.0 (s, 1H, -N=CH), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.83 (d, 2H, Ar-H), 7.52 (m, 3H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 166.7(C=O), 153.6(C=N), 166.7(N=CH), 21.3(CH3), 126.6, 127.3, 133.4, 126.7, 148.7, 120.8, 131.8, 116.5, 121.2, 151.6, 126.7, 130.3, 132.4, 138.2, 133.7, 129.2, 128.8, 131.0 (aromatic); MS (m/z, (relative abundance, %)): 495 (M+, 0.68), 397 (26.85), 284 (24.6), 176 (51.2), 115 (10.1), 85 (11.31).Synthesis of 4-(3-(3–chloro–2–oxo–4–phenylazetidin–1- yl)–4–oxo–3,4–dihydroquinazolin–2–yl)phenyl–4–methyl benzene sulfonate (14).A mixture of 13 (4.95 g, 0.01 mole), chloroacetyl chloride (1.13 g, 0.01 mole) and triethyl amine (5 drops) in dry dioxane (30 mL) was heated under reflux for 8 hours. The solid product that separated on cooling was filtered off, dried and recrystallized from ethanol to give 14.White crystals; Yield 42%; m.p. 115-117℃; Anal. Calcd. for C30H22CIN3O5S: C, 62.99; H, 3.85; N, 17.14. Found: C, 62.81; H, 3.64; N, 17.12; IR (KBr, cm-1): 1678 (CON), 1596 (C=N), 1360 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 4.1 (s, 1H, CH), 3.5 (s, 1H, CH), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.29 (d, 2H, Ar-H), 7.45 (t, 2H, Ar-H), 7.27 (t, 1H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 160.7, 163.6(2C=O), 156.2(C=N), 70.6(N-CH), 76.6(CH-Cl), 21.3(CH3), 126.6, 127.3, 133.4, 126.7, 148.7, 120.8, 131.8, 116.5, 121.2, 151.6, 126.7, 130.3, 132.4, 138.2, 143.5, 126.9, 128.5, 126.7 (aromatic); MS (m/z, (relative abundance, %)): 571 (M+, 12.55), 453 (30.45), 365 (100), 247 (33.1), 175 (17.1), 112 (23.1).Synthesis of 4–(4– oxo –3–(4– oxo–2–phenyl thiazolidin –3–yl)-3,4-dihydroquinazolin–2–yl)pheny-4–methyl benzenesulfonate (15).Thioglycolic acid (1.50 g, 0.01 mole) in dry benzene (20 mL) was added drop wise with stirring at room temperature to 13 (4.95 g, 0.01 mole) in dry benzene (20 mL) for 1 hour. The reaction mixture was heated under reflux for 6 hours, cooled and the precipitated product was filtered off and recrystallized from ethanol to give the desired product 15.Yellow crystals; Yield 47%; m.p. 80-85℃; Anal. Calcd. for C30H23N3O5S2: C, 63.27; H, 4.04; N, 17.07. Found: C, 63.15; H, 4.00; N, 17.02; IR (KBr, cm-1): 1678 (CON), 1596 (C=N), 1360 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 4.9 (s, 1H, CH), 3.9 (s, 2H, CH2), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.36 (d, 2H, Ar-H), 7.33 (t, 2H, Ar-H), 7.27 (t, 1H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H); 13C-NMR(400 MHz, DMSO-d6, δ / ppm): 160.7, 163.6(2C=O), 156.2(C=N), 35.6(CH2-S), 62.2(CH-S), 21.3(CH3), 126.6, 127.3, 133.4, 126.7, 148.7, 120.8, 131.8, 116.5, 121.2, 151.6, 126.7, 130.3, 132.4, 138.2, 128.6, 126.9, 127.1, 139.2 (aromatic); MS (m/z, (relative abundance, %)): 569 (M+, 10.34), 481 (5.45), 397 (23.18), 288 (51.2), 185 (100), 91 (24.2).Synthesis of 4-(3,3–diacetamido–4–oxo–3,4–dihydro quinazolin–2–yl) phenyl-4- methylbenzenesulfonate (16) and 4-(4–oxo–3,3–(dibenzamido)–3,4–dihydroquinazolin –2–yl)phenyl– 4– methyl benzenesulfonate (17). A solution of quinazolinone 12 (4.07 g, 0.01 mole), acetyl chloride (1.56 g, 0.02 mole) and/or benzoyl chloride (2.8 g, 0.02 mole) in dry pyridine (30 mL) was heated under reflux for 3 hours. The reaction mixture was cooled, then poured over ice/ HCl and the solid that separated out was filtered off, washed with water several times, dried and then recrystallized from methanol to afford 16 and 17, respectively. Compound 16White crystals; Yield 82%; m.p. 145-147℃; Anal. Calcd. for C25H21N3O6S: C, 61.10; H, 4.28; N, 14.73. Found: C, 61.24; H, 4.16; N, 14.72; IR (KBr, cm-1): 1701-1736 (N-C=O), 1653 (CON), 1597 (C=N), 1360 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 2.27 (s, 6H, -COCH3), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H);13C-NMR(400 MHz, DMSO-d6, δ / ppm): 160.7, 156.6(3C=O), 156.2(C=N), 21.3, 20.8(3CH3), 126.6, 127.3, 133.4, 126.7, 148.7, 120.8, 131.8, 116.5, 121.2, 151.6, 126.7, 130.3, 132.4, 138.2 (aromatic); MS (m/z, (relative abundance, %)): 491 (M+, 5.47), 386 (23.45), 285 (11.8), 168 (100), 122 (13.5), 91 (12.4). Compound 17White crystals; Yield 84%; m.p. 160-162℃; Anal. Calcd. for C35H25N3O6S: C, 68.29; H, 4.07; N, 18.45. Found: C, 68.09; H, 4.00; N, 18.42; IR (KBr, cm-1): 1701-1736 (N-C=O), 1653 (CON), 1597 (C=N), 1360 (SO3);13C-NMR(400 MHz, DMSO-d6, δ / ppm): 160.7, 172.0(3C=O), 156.2(C=N), 21.3(CH3), 126.6, 127.3, 133.4, 126.7, 148.7, 120.8, 131.8, 116.5, 121.2, 151.6, 126.7, 130.3, 132.4, 138.2, 127.5, 128.8, 132.1, 134.3 (aromatic); MS (m/z, (relative abundance, %)): 615 (M+, 5.47), 566 (3.65), 376 (100), 288 (11.45), 182 (3.8), 113 (56.4).Synthesis of 4–(3–(1,3–dioxoisoindolin-2–yl)–4–oxo –3,4– dihydroquin-azolin–2–yl) phenyl–4–methylbenzene sulfonate (18). A mixture of quinazolinone 12 (4.07 g, 0.01 mole) and phthalic anhydride (1.48 g, 0.01 mole) was fused in an oil bath at 150-160C for 6 hours. The reaction mixture was triturated with ice/HCl. The solid product was filtered off, washed with water several times, dried and then recrystallized from ethanol affording 18.Brown crystals; Yield 65%; m.p. 175-177℃; Anal. Calcd. for C29H19N3O6S: C, 64.80; H, 3.54; N, 16.11. Found: C, 64.66; H, 3.39; N, 16.12; IR (KBr, cm-1): 1744-1797 (2 C=O), 1704 (CON), 1601 (C=N), 1376 (SO3); 1H-NMR (500 MHz, DMSO-d6, δ / ppm): 2.4 (s, 3H, CH3), 8.03 (d, 1H, Ar-H), 7.65 (t, 1H, Ar-H), 7.70 (t, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.85-7.88 (m, 4H, Ar-H), 7.40 (d, 2H, Ar-H), 7.02 (d, 2H, Ar-H), 7.74 (d, 2H, Ar-H), 7.42 (d, 2H, Ar-H);13C-NMR(400 MHz, DMSO-d6, δ / ppm): 160.7, 164.0(3C=O), 156.2(C=N), 21.3(CH3), 126.6, 127.3, 133.4, 126.7, 148.7, 120.8, 131.8, 116.5, 121.2, 151.6, 126.7, 130.3, 132.4, 138.2, 132.0, 123.7, 132.2 (aromatic); MS (m/z, (relative abundance, %)): 537 (M+, 35.45), 389 (34.45), 275 (23.28), 178 (100), 145 (17.7), 95 (22.3).
4.1. Microbiological procedures for the activity study.
4.1.1. Materials and method
Media: Nutrient agar and Potato Dextrose Agar[6,7] plates were used for bacterial and fungal organisms respectively.
4.1.2. Preparation of microbial suspension
The bacterial and fungal strains were subculture at 37℃ for six hrs in the corresponding medium of three successive days. These suspensions were used to insulate the antibiograms.
4.1.3. Preparation of the biograms
The agar disk diffusion method was performed on each of the tested substance solution in dimethylformamide. Filter paper discs were impregnated with 1 ml of the solution and placed on the inoculated plates. These plates after standing at 4℃ for 2 hours were incubated at 37℃ for 24 hours. The diameters of the inhibition zones were measured in millimeters.
5. Conclusions
A series of novel substituted quinazolone derivatives were synthesized by the reaction of benzoxazinone derivative 2 with some primary aromatic amines. All the compounds were subjected to biological screening and they showed promising antibacterial and antifungal activity which were comparable to the activity of known standard drugs where compounds 3, 5, 7, 8, 9, 10, 11, 17 and 18 exhibited good activities against the reference chemotherapeutics due to the presence of antipyrine, pyridine and phthalimide moieties which play an important as antibacterial and antifungal, while 8, 9, 10 and 18 exhibited good activities against Bacillus Thuringenesis and 5, 10, 11, 14, 15, 17 and 18 exhibited good activities against Klebseilla Pneumonia. On the other hand, the results for antifungal activities revealed that, compounds 5, 6, 7, 16 and 17 exhibited good activities against Trichoderma Herzianum and Trichoderma Virdi. This proves the high therapeutic value of these compounds and encourages further study to explore their biological potential.
References
| [1] | K. Waisser, E. Petrlíková, M. Peřina, V. Klimešová, J. Kuneš, K. Palát, J. Kaustová, H. Dahse, U. Möllmann, European Journal of Medicinal Chemistry, 45, 2719-2725, 2010. |
| [2] | X. Li, N. Liu, H. Zhang, S. E. Knudson, R. A. Slayden, P. J. Tonge, Bioorganic & Medicinal Chemistry Letters, 20, 6306-6309, 2010. |
| [3] | D. Zhou, L. B. Harrison, U. Shah, H. T. Andree, A. G. Hornby, R. Scerni, E. L. Schechter, L. D. Smith, M. K. Sullivan, E. R. Mewshaw, Bioorg. Med. Chem . Lett. 16, 1338, 2006. |
| [4] | O. M. O. Habib, E. B. Moawad, S. D. Badawy, J. A. F. Mansour, J. Prakt. Chem. 332, 791, 1990. |
| [5] | D. J. Wesley, H. E. Henry, J. Am. Chem. Soc. 78, 2543, 1956. |
| [6] | A. A. El-Khamry, S. El-Nagdy, E. M. Shaban, Egypt J. Chem. 31, 241, 1988. |
| [7] | S. R. Verma, L. W. Nobles, J. Pharm. Sci. 57, 1801, 1968. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML