-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(2): 1-6
doi: 10.5923/j.ajoc.20120202.01
SBSA as a New and Efficient Catalyst for the One-Pot Green Synthesis of Benzimidazole Derivatives at Room Temperature
Sami Sajjadifar , Seyed Ahmad Mirshokraie , Nematollah Javaherneshan , Omid Louie
Department of Chemistry, Payame Noor University, PO BOX 19395-4697 Tehran, Iran
Correspondence to: Sami Sajjadifar , Department of Chemistry, Payame Noor University, PO BOX 19395-4697 Tehran, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
silica boron sulfonic acid (SBSA) was easily prepared and used as a new and efficient solid acid catalyst for the synthesis of benzimidazole derivatives with high isolated yields. Various substituted benzimidazoles were synthesized by a combination of o-phenylenediamines and aldehydes in the presence of boron sulfonic acid in with good yields in water and under a mild reaction conditions. This method is also applicable for precursors such as: aromatic and unsaturated aldehydes and o-phenylenediamines.
Keywords: Silica Boron Sulfonic Acid, SBSA, Solid Acid, Benzimidazole Synthesis, Drugs, Green Synthesis
Article Outline
1. Introduction
- Benzimidazole moieties are classified under several classes of drugs[1], based on the possible substitution at different positions of the benzimidazole nucleus. Benzimidazole derivatives exhibit significant activity against several viruses such as HIV, human cytomegalovirus (HCMV)[2], herpes (HSV-1)[3], RNA[4] and influenza[4]. Furthermore they have been also used to act as topoisomerase inhibitors[6], selective neuropeptide YY1 receptor antagonists[7], angiotensin II inhibitors[8], potential antitumor agents[9] and smooth muscle cell proliferation inhibitors[10]. In addition benzimidazoles are very important precursors in organic synthesis. Vitamin B12 constitutes a milestone in the chemistry of benzimidazoles. Bisbenzimidazole is DNA- minor grove binding agents possessing anti-tumour activity[11].A number of methods have been reported for the synthesis of benzimidazoles such as the condensation of o-aryldiamines and aldehyde in refluxing nitrobenzene[12]. The coupling of phenylenediamines and carboxylic acids [13] or their derivatives (nitriles, imidates, or orthoesters)[14], which often requires strong acidic conditions[15], and sometimes combines with very high temperatures or microwave irradiation[16]. The other route involves a two-step procedure that includes the oxidative cyclo- dehydrogenation of Schiff bases, which are often generated from the condensation of o- phenylenediamines and aldehydes. Dir - condensation of o-aryldiamines and aldehydes is not a good synthetic reaction, as it is well known to yield a complex mixture, being 1,2-disubstituted benzimidazoles, the bis anil and dihydrobenzimidazoles as the main side products[17]. However, the addition of transition metal, namely copper (II) acetate[18], mercury oxide[19] or lead tetracetate[20] allows a partial selective synthesis of benzimidazoles. In recent years, solvent-free synthesis of benzimidazoles under microwave irradiation using Yb(OTf)3[21], KSF clay[22], PPA[23], Na2SO4[24], K-10 clay[25], metal halide supported alumina[26] and solid support[27] have been reported. Various oxidative and catalytic reagents such as sulfamic acid[28], I2[29], DDQ[30], Air[31], Oxone[32], FeCl3·6H2O[33], In(OTf)3 [34], Yb(OTf)3[35], Sc(OTf)3[36], KHSO4[37], IL[38], Nitrobenzene[39] , 1,4 – benzo quinine [40], tetracyano ethylene [41], benzofuroxan [42], MnO2 [43], Pb(OAc)4[44], NaHSO3[45], Na2S2O5[46], DMP[47], NH4VO3[48], have been employed. Benzimidazole derivatives can be synthsised by another catalysts such as CAN [49], p-TsOH[50], BE3.OEt2[51], KHSO4[52], CuPy2Cl2 [53], polyphosphoric acid[54], mineral acids[55], boric acid [56], p-TSA [57], Dowex 50W [58], SSA [59], solid acid scolecite [60], YCl3 [61], Zn(OAc)2 [62], N- halosuccinamide (X = Cl, Br, I) [63], Yb(OTf)3 [64], PEG-100 [65], (NH4)H2PW12O40 [66], bismuth chloride[67], mercury chloride[68], Ionic liquids[69], AMA[70], TBAF[71], H2O2/ SiO2-FeCl3[72], HBF4-SiO2[73] and MoO3/CeO2-ZrO2[74]. Unfortunately, many of these processes suffer some limitations, such as drastic reaction conditions, low yields, tedious work up procedures and co-occurrence of several side reactions. In this article, we report a simple and efficient method for the synthesis of benzimidazole derivatives using SBSA as a catalyst under mild reaction conditions. We used water as a green solvent. Water as a green reaction medium is highly appreciated. As a solvent, water possesses the following distinct advantages of being safe, nonflammable, readily available in large quantities, operationally very simple and devoid of any carcinogenic effects. Therefore, water mediated organic reactions for the preparation of biologically active molecules constitutes a major challenge for chemists involved in organic synthesis.Firstly, BSA was introduced by Kiasat et al (Scheme 1) and used for the regioselective conversion of epoxides to thiocyanohydrins under solvent-free reaction conditions[75]. We converted it to SBSA catalyst by using silica gel. We are investigating applications of this catalyst in organic synthesis.
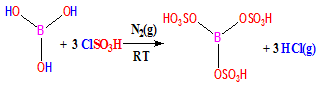 | Scheme 1. |
2. Methods
2.1. General
- IR spectra of the compounds were obtained on a Shimadzu IR-435 spectrometer using a KBr disk. The 1H nuclear magnetic resonance (1H NMR) spectra were recorded on a Bruker AQS 300 Avance instrument at 300 MHz in dimethyl sulfoxide (DMSO-d6) using tetramethylsilane as an internal standard. The progress of reaction was followed with thin-layer chromatography (TLC) using silica gel SILG/UV 254 and 365 plates. All the products are known compounds and were characterized by comparing the IR, 1H NMR, and 13C NMR spectroscopic data and their melting points with the literature values.
2.2. Typical Procedure for the Synthesis of Benzimidazoles
- A mixture of o-phenylenediamine derivatives 1 (1 mmol), aromatic aldehyde 2 (1 mmol), and SBSA (0.05g, 5 mol %) in 10 mL of water, was stirred in a round bottomed flask at room temperature for 30 minutes (Table 2). The progress of the reaction was followed by TLC. After completion of the reaction, the reaction mixture was added dropwise with vigorous stirring into a mixture of Na2CO3 (0.106g, 0.1 mmol) and H2O (20 mL). In cases where the product precipitated as a free flowing solid, it was collected by filtration, washed with H2O and dried. In cases where gummy material precipitated the product was extracted into EtOAc, the organic phace was washed with H2O and dried with Na2SO4. Evaporation of the solvent gave the crude product, which was purified by column chromatography over silica gel (n-hexane:ethyl acetate, 5:1) to afford the corresponding benzimidazole. All of the compounds are known compounds which they were identified from their 1H NMR spectroscopic data and by comparing their melting points with those reported in the literature[17-20,49,51,61,62, 76,77].
2.3. Preparation of silica boron sulfonic acid (SBSA) [75]
- A 50 mL suction flask was equipped with a constant pressure dropping funnel. The gas outlet was connected to a vacuum system through water adsorbing solution and an alkali trap. Boric acid (1.55 g, 25 mmol) was charged in the flask and chlorosulfonic acid (8.74 g, ca. 5 mL, 75 mmol in 5 ml CH2Cl2) was added dropwise over a period of 1 h at room temperature under N2(g). Hydrogen chloride evolved immediately. After completion of the addition, the mixture was shaken for 85 min, while the residual HCl was eliminated by suction. Then the mixture was washed with diethyl ether to remove the unreacted chlorosulfonic acid (1HNMR of SBSA in Acetone-D6 show δ=12.218) and then add 14.4 g silica gel and stirred those. Finally, dried and grayish solid material was obtained (21.6 g, 95.66%).
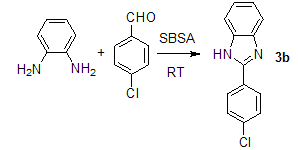 | Scheme 2. |
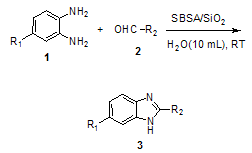 | Scheme 3. Synthesis of 1,3-benzimidazole derivatives 3 from the reaction of o-phenylenediamines with various aldehydes. The reasonable mechanism as shown below |
|
3. Results and Discussion
- In continuation of our studies on sulfonic acid based catalysts such as silica sulforic acid (SSA), silica chloride, silica phosphoric acid, 1,3,5-Triazine-2,4,6-triyltrisulfamic acid (TTSA), ionic liquid with sulfonic acid moieties and so on, we decide to use boron sulfonic acid(SBSA) for the synthesis of substituted benzimidazoles. Firstly, condensation of o-phenylenediamine and benzaldehyde was performed with different molar ratio of SBSA, solvents and temperatures to optimize the reaction conditions. Various solvents with a good range of molar ratios of the catalyst were employed and the results are depicted in Table 1. As shown in Table 1, a mixture of 5 mol% of SBSA in H2O (10 mL) created the best reaction media and afforded the benzimidazole 3b with optimum yields among the conditions tested (entry 9, Table 1). In order to find a suitable catalyst ratio for the synthesis of benzimidazoles from 1,2-diamines and aldehydes, the condensation of benzene-1,2-diamine with 4-chlorobenzaldehyde was chosen as a model to provide compound 3b (Scheme 2).Herein, we wish to report a novel protocol for the rapid synthesis of a variety of biologically significant benzimidazoles using a catalytic amount of SBSA under mild aqueous conditions (Scheme 3). The reaction was carried out in neat at room temperature for 30 minutes, using o-phenylenediamine (1 mmol) and aldehyde (1 mmol) in the presence of SBSA (0.05 mmol). The results are summarized in Table 2.
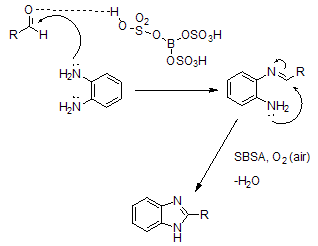 | Scheme 4. Mechanisms for the synthesis of 1- and 2-substitute-1,3- benzimidazoles 3 via condensation reaction of phenylenediamines with different aldehydes |
4. Conclusions
- In conclusion, we have developed a one-pot, simple and efficient method for the synthesis of 2-arylsubstituted benzimidazoles by the condensation of o-phenylenediamine with arylaldehyde catalyzed by SBSA. As shown in Table 2, a wide variety of aromatic compounds and α,β-unsaturated aldehydes having both electron- donating and electrone withdrawing groups and substituted o-phenylenediamine react to give the corresponding benzimidazole in good yields. Best results were obtained using 0.15 equivalents of SBSA, lower loading resulted in lower yields, while higher loading did not increase product yields significantly. This method offers several advantages such as high conversions, shorter reaction times, non-toxic cost efficiency providing, recyclability of the catalyst, cleaner reaction profiles and simple experimental and work-up procedures. In summary, a simple work-up procedure, mild reaction conditions and very good yields make our methodology a valid contribution to the existing processes in synthesis of benzimidazole derivatives. The aliphatic aldehydes which also were not reacted under similar conditions gave considerable yields (Table 2, entries 3o-q).
ACKNOWLEDGEMENTS
- The authors thank Payame Noor University of ilam for financial support of this work.
References
| [1] | J. Velik, V. Baliharova, J. Fink-Gremmels, et al., Res. Vet. Sci., 2004, 76, 95. Spasov, A. A.; Yozhitsa, I. N.; Bugaeva, I. I.; Anisimova, V. A., Pharm. Chem. J. ,1999, 33, 232. |
| [2] | Porcari, A. R.; Devivar, R. V.; Kucera, L. S.; Drach, J. C. ; Townsend, L. B. J. Med. Chem. 1998, 41, 1252. Roth, T.; Morningstar, M. L.; Boyer, P. L.; Hughes, S. H.; Buckheitjr, R. W.; Michejda, C. J. J. Med. Chem. 1997, 40, 4199. |
| [3] | Migawa, M. T.; Girardet, J. L.; Walker, J. A.; Koszalka, G. W.; Chamberlain, S. D.; Drach, J. C., Townsend, L. B., J. Med. Chem., 1998, 41, 1242. |
| [4] | Tamm, I.; Sehgal, P. B., Adv. Virus Res., 1978, 22, 187. |
| [5] | Tamm, I., Science, 1957, 126, 1235. |
| [6] | Kim, J. S.; Gatto, B.; Yu, C.; Liu, A.; Liu, L. F.; Lavioe, E. J. Med. Chem. 1996, 39, 992. |
| [7] | Zarrinmayeh, H.; Zimmerman, D. M.; Cantrell, B. E.; Schober, D. A.; Bruns, R. F. Bioorg. Med. Chem. Lett. 1999, 9, 647. |
| [8] | Kohara, Y.; Kubo, K.; Imamiya, E.; Wada, T.; Inada, Y.; Naka, T. J. Med. Chem. 1996, 39, 5228. |
| [9] | Denny, W. A.; Rewcastle, G. W.; Bagley, B. C. J. Med. Chem. 1990, 33, 814. |
| [10] | Elokdah, H. M.; Chai, S. Y.; Sulkowski, T. S. US Patent, 1998, 5 764 473; Chem. Abstr. 1998, 129, 58784g. |
| [11] | J. Mann..; A.Baron; Y Opoku-Boahen.; E Johansoon.; G.Parkmson: L. R Kelland.; S Neidle., J. Med. Chem., 2001, 44, 138. |
| [12] | (a) B. Yadagiri, J. W. Lown, Synth. Commun., 1990, 20, 955. (b) Q. Sun, B. Yan, Bioorg. Med. Chem. Lett., 1998, 8, 361. |
| [13] | (a) Hisano, T.; Ichikawa, M.; Tsumoto, K.; Tasaki, M. Chem. Pharm. Bull. 1982, 30, 2996. |
| [14] | (b) Czarny, A.; Wilson, W. D.; Boykin, D. W. J. Heterocycl. Chem. 1996, 33, 1393 and Tidwell, R. R.; Geratz, J. D.; Dann, O.; Volz, G.; Zeh, D.; Loewe, H. J. Med. Chem. 1978, 21, 613. |
| [15] | P. N. Preston, Benzimidazoles and Congeneric Tricyclic Compounds, In The Chemistry of Heterocyclic Compounds, Part 1, Vol. 40, Eds.: Weissberger, A.; Taylor, E. C., Wiley: New York, 1981, pp. 6-60. M. R. Grimmett, Imidazoles and their Benzo Derivatives, In Comprehensive Heterocyclic Chemistry, Vol. 5, Eds.: Katritzky, A. R.; Rees, C.W., Pergamon: Oxford, 1984, pp. 457-487. T. Benincori, F. Sannicolo, J. Heterocycl. Chem., 1988, 25, 1029. |
| [16] | Reddy, G. V.; Rao, V. V. V. N. S. R.; Narsaiah, B.; Rao, P. S. Synth. Commun. 2002, 32, 2467. Perumal, S.; Mariappan, S.; Selvaraj, S. Arkivoc 2004, 8, 46. Rao, A.; Chimirri, A.; Ferro, S.; Monforte, A. M.; Monforte, P.; Zappalà, M. Arkivoc 2004, 5, 147. |
| [17] | J. G. Smith, I. Ho, Tetrahedron Lett., 1971, 38, 3541. |
| [18] | R. Weidenhagen, Ber., 1936, 69, 2263. |
| [19] | P. Jakobson, M. Jannicke, F. Meyer, Ber., 1896, 29, 2682. |
| [20] | F. F. Stevens, J. D. Bower, J. Chem. Soc., 1949, 2971. |
| [21] | L. Wang, J. Sheng, H. Tian, et al., Synth. Commun., 2004, 34, 4265. |
| [22] | A. Loupy, A. Petit, J. Hamelin, et al., Synthesis, 1998, 9, 1213. |
| [23] | J. Lu, B. Yang, Y. Bai, Synth. Commun., 2002, 32, 3703. |
| [24] | M.P. Surpur, P.R. Singh, S.B. Patil, et al, Synth. Commun., 2007, 37, 1375. |
| [25] | S. Perumal, S. Mariappan, and S. Selvaraj, ARKIVOC, 2004 (viii) 46-51. |
| [26] | G. V. Reddy, V. V. V. N. S. Ramarao, B. Narsaiah, et al, Synth. Commun., 2002, 32, 2467. |
| [27] | G. Penieres, I. Bonifas, G. Lopez, et al, Synth. Commun., 2000, 30, 2191. K. Bougrin, A. Loupy, A. Petit, et al, Tetrahedron, 2001, 57, 163. |
| [28] | Chakrabarty, M.; Karmakar, S.; Mukherji, A.; Arima, S.; Harigaya, Y., Heterocycles 2006, 68,967. |
| [29] | Gogoi, P.; Konwar, D., Tetrahedron Lett., 2006, 47, 79. |
| [30] | Lee, K. J.; Janda, K. D. Can. J. Chem. 2001, 79, 1556. |
| [31] | Lin, S.; Yang, L. Tetrahedron Lett., 2005, 46, 4315. |
| [32] | Beaulieu, P. L.; Hache, B., Von Moos, E. Synthesis, 2003, 1683. |
| [33] | Singh, M. P; Sasmal, S.; Lu, W.; Chatterjee, M. N. Synthesis, 2000, 1380. |
| [34] | Trivedi, R.; De, S. K.; Gibbs, R. A. J. Mol. Cat. A: Chem., 2005, 245 , 8. |
| [35] | Massimo, C.; Francesco, E.; Francesca, M., Synlett., 2004, 1832. |
| [36] | Itoh, T.; Nagata, K.; Ishikawa, H., Ohsawa, A. Heterocycles, 2004, 63, 276. Nagata, K.; Itoh, T.; Ishikawa, H., Ohsawa,A. Heterocycles, 2003, 61, 93. |
| [37] | Ma, H. Q.; Wang, Y. L.; Wang J. Y., Heterocycles 2006, 68, 1669. |
| [38] | Ma, H. Q.; Wang, Y. L.; Li, J. P.; Wang, J. Y Heterocycles 2007, 71, 135. |
| [39] | Dubey, P. K.; Ratnam, C. V. Indian J. Chem. B 1979, 18, 428. |
| [40] | Verner, E.; Katz, B. A.; Spencer, J. R.; Allen, D.; Hataye, J.; Hruzewicz, W.; Hui, H. C.; Kolesnikov, A.; Li, Y.; Luong, C.; Martelli, A.; Radika, K.; Rai, R.; She, M.; Shrader, W.; Sprengeler, P. A.; Trapp, S.; Wang, J.; Young, W. B.; Mackman, R. L. J. Med. Chem. 2001, 44, 2753. Kumar, S.; Kansal, V.; Bhaduri, A. Indian J. Chem. B 1991, 20, 254. |
| [41] | Vanden Eynde, J. J.; Delfosse, F.; Lor, P.; Van Haverbeke, Y., Tetrahedron, 1995, 51, 5813. Lee, K. J.; Janda, K. D. Can. J. Chem. 2001, 79, 1556. Chikashita, H.; Nishida, S.;Miyazaki, M.;Morita-Itoh, Y. K. Bull. Chem. Soc. Japan. 1987, 60, 737. |
| [42] | Patzold, F.; Zeuner, F.; Heyer, T. H.; Niclas, H. J., Synth. Commun., 1992, 22, 281. |
| [43] | Bhatnagar, I.; George, M. V., Tetrahedron, 1968, 24, 1293. |
| [44] | Stephens, F. F.; Bower, J. D., J. Chem. Soc., 1949, 2971. |
| [45] | Weidner-Wells, M. A.; Ohemeng, K. A.; Nguyen, V. N.; Fraga-Spano, S.; Macielag, M. J.; Werblood, H. M.; Foleno, B. D.; Webb, G. C.; Barrett, J. F.; Hlasta, D. J. Bioorg. Med. Chem. Lett. 2001, 11, 1545. Austen, S. C.; Kane, J. M.; J. Heterocycl. Chem. 2001, 38, 979. |
| [46] | Lombardy, R. L.; Tanious, F. A.; Ramachandran, K.; Tidwell, R. R. J. Med. Chem. 1996, 39, 1452. Xiao-Hang Zhang et al, Bull. Korean Chem. Soc., 2007, 28(8), 1389-1394. |
| [47] | Dabhade, S. K.; Bora, R. O.; Farooqui, M.; Gill, C. H. Chin. Chem. Lett. 2009, 20(8), 893. |
| [48] | Jadhav, G. R.; Shaikh, M. U.; Kale, R. P.; Gill, C. H. Chin. Chem. Lett. 2009, 20, 292. |
| [49] | Rajesh Kumar and Y. C. Joshi, e-journal of Chemistry, 2007,4(4), 606-610. |
| [50] | Han Xiangming, Ma Huiqiang and Wang Yulu, ARKIVOC, 2007 (xiii) 150-154. |
| [51] | Rahul R. NAGAWADE, Devanand B. SHINDE, Chinese Chemical Letters, 2006, 17, 4, 453-456. |
| [52] | Ma, H. Q., Wang, Y. L., Wang J. Y., Heterocycles, 2006, 68, 1669. |
| [53] | J. Venu Madhav, B. Suresh Kuarm, and B. Rajitha, ARKIVOC, 2008 (xiii), 145-150. |
| [54] | Preston, P. N. Benzimidazoles,and Congeneric Tricyclic Compounds, In Weissberger, A.; Taylor, E. C. Eds., Chemistry of Heterocyclic Compounds. Part 1, vol. 40, Wiley, New York, 1981, pp 6-60. |
| [55] | Grimmet, M. R. Imidazoles and their benzoderivatives, In Kartitzky, A. R.; Rees, C. W. Eds., Comprehensive Heterocyclic Chemistry, Vol. 5. Pergamon: Oxford, 1984, p. 457. |
| [56] | Pushkina, L. N.; Mazalov, S. A.; Poatovskii, I. Ya. Zh. Obshch. Khim. 1962, 32, 2624; Chem Abstr. 1963, 58, 9049h. |
| [57] | Yoshiyuki, T.; Kazuaki, Y. Hokkaido Daigaku Koagakubu Kenkyu Hokoku 1980. Chem Abstr., 1980, 93, 45-49204537k. |
| [58] | Chhanda Mukhopadhyay et al, ARKIVOC, 2009 (xiii) 1-22. |
| [59] | Peyman Salehi et al, Tetrahedron Letters, 2006, 47, 2557-2560. |
| [60] | L. S. Gadekar et al, Chinese Chemical Letters, 2010, 21, 1053–1056. |
| [61] | Ch. Siva Subrahmanyam and S. Narayanan, IJABPT, 2010, 1(2), 789-794(www.ijabpt.com). |
| [62] | V. D. Patil et al, Der Chemica Sinica, 2010, 1 (2), 125-129(www.pelagiaresearchlibrary.com). |
| [63] | H Fujioka.; K Murai.; Y Ohba..; A Hiramastsu.; Y Kita.. Tetrahedron Lett., 2005, 46, 2197. |
| [64] | D. S Van Vliet.; P Gillespie.; J Scicinski J., Tetrahedron Lett., 2005, 46, 6741. |
| [65] | Chhanda Mukhopadhyay ; Pradip Kumar Tapaswi,. Tetrahedron lett., 2008, 49, 6237. |
| [66] | B. Y Giri. et al, Synthetic commun., 2007, 3, 2331. |
| [67] | Y.-S. Su, Ch.-M. Sun, Synlett., 2005, 1243. |
| [68] | Y.-S. Su, M.-J. Lin, M.Ch. Sun, Tetrahedron Lett., 2004, 46, 177. |
| [69] | R.N. Nadaf et al, J. Mol. Catal. A: Chem., 2004, 214, 155. |
| [70] | K. Niknam and A. Fatehi-Raviz, J. Iran. Chem. Soc., 2007, 4(4), 438-443. |
| [71] | R. S. Joshi et al, JCCS, 2010, 57, 1227-1231. |
| [72] | Abbas Fazlinia et al, JKCS, 2010, 54(2), 579-581. |
| [73] | Abasaheb V. Patil et al, 2010, Bull. Korean Chem. Soc., 2010, 31(6), 1719-1722. |
| [74] | Sandip B. Rathod et al, Bull. Korean Chem. Soc., 2010, 31(10), 2835-2840. |
| [75] | A. R. Kiasat, M. Fallah-Mehrjardi, J. Braz. Chem. Soc., 2008, 19(8), 1595-1599. |
| [76] | Abdelkrim Ben Alloum, Khalid Bougrin and Mohamed Soufiaoui, Tetrahedron Letters, 2003, 44, 5935–5937. |
| [77] | Mazaahir Kidwai, Anwar Jahan and Divya Bhatnagar, J. Chem. Sci., 2010, 122(4), 607–612. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML