-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(1): 28-34
doi:10.5923/j.ajoc.20120201.06
New Thiazolidinones, Thiazolines and Thiopyrimidines from 3,5-Diphenylcyclohex-2-enone
M. A. Metwally, E. Abdel-Galil, F. A. Amer, A. M. Abdallah
Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
Correspondence to: M. A. Metwally, Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
1-(3,5-Diphenylcyclohex-2-enylidene)hydrazine 3 was prepared and used as a key intermediate for the synthesis of thiazolidin-5-one 6, thiazolidn-4-one 7, thiazolines 8a,b, 9 and thioxodihydropyrimidine 10. Base catalyzed Knoevenagel condensation of compounds 6, 7 and 10 with different aldehydes gave the arylidene derivatives 11a,b, 12a,b and 13a,b respectively. Treatment of compounds 6, 7 and 10 with different aromatic diazonium salts gave azodye derivatives 14a,b, 15a,b and 16a,b. The newly synthesized compounds were characterized by IR, 1H-NMR and mass spectral data.
Keywords: Thiazoloidinones, Thiazolines and Thiopyrimidines
Cite this paper: M. A. Metwally, E. Abdel-Galil, F. A. Amer, A. M. Abdallah, New Thiazolidinones, Thiazolines and Thiopyrimidines from 3,5-Diphenylcyclohex-2-enone, American Journal of Organic Chemistry, Vol. 2 No. 1, 2012, pp. 28-34. doi: 10.5923/j.ajoc.20120201.06.
1. Introduction
- In recent years, thiazolidinones and their derivatives have become among the most extensively investigated compounds. They constitute an important five-membered heterocycles, having valuable biological activities in the areas of medicine and agriculture[1]. They have found uses, for example, as bactericidal[2,3], fungicidal[4,5], insecticidal [6,7], anticonvulsant[8-11], tuberculostatic[12,13], herbicidal [14,15], antiviral[16,17], antiprotozoal[18], antimalarial[19], antimicrobial[20,21], anti- inflammatory[22,23], antitumor[24], antitubercular agents[25] and specially as anti-HIV agents[26]. The diversity of biological and phy- siological activities of organic sulfur heterocycles may be attributed to the presence of the NCS fragment, which is characteristicof thiazoles, thiazolines, and thiazolidines[27]. In view of the above findings and as an extension of our studies[28-34] aiming to the synthesis of different thiazoles, thiazolodinones and thiazolines, of expected pharmaceutical interest, we report here the reactivity of potassium N’ -3,5 - diphenylcyclohex-2-enylidene-N-phenylcarbamoyl hydraz- onothionate 4 toward different α-halogenated compounds.
2. Results and Discussion
- The key intermediate, 3 required for the synthesis of the title compounds was prepared according to the procedure outlined in the Scheme 1. For the synthesis of 3, reaction sequence including the first step decarboxylation of ethyl 2-oxo-4,6-diphenylcyclohex-3-ene carboxylate 1 either by a previously reported procedure [35] with NaOH solution or our new methodology using acetic acid gave3,5- diphenylcyclohex-2-enone 2. This was reacted with hydrazine hydrate to afford 1-(3,5-diphenylcyclohex-2-enylidene) hydrazine 3 in excellent yield. The structures of the synthesized compounds (2 and 3) were confirmed by IR, 1H-NMR and mass spectral analyses. The IR spectrum of compound 2 no absorption was appeared for the ester group. The mass spectrum of 2 showed the molecular ion peak at m/z= 248 (M+, 100%) which is equivalent to the molecular formula (C18H16O). The mass spectrum of compound 3 showed the molecular ion peak at m/z = 262 (M+, 100%), corresponding to the molecular formula (C18H18N2).
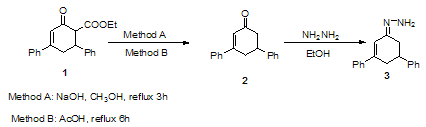 | Scheme 1. |
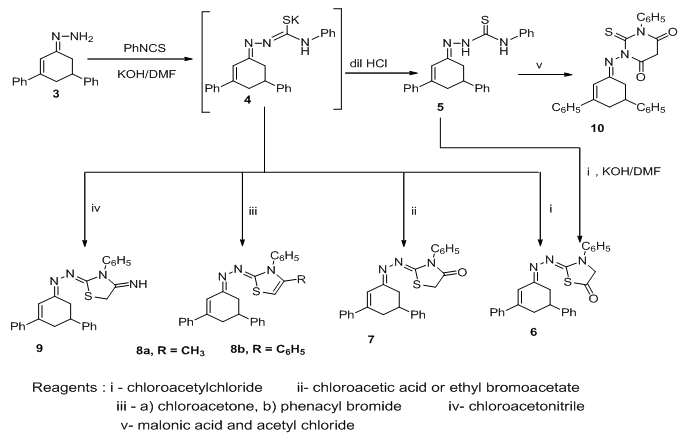 | Scheme 2. |
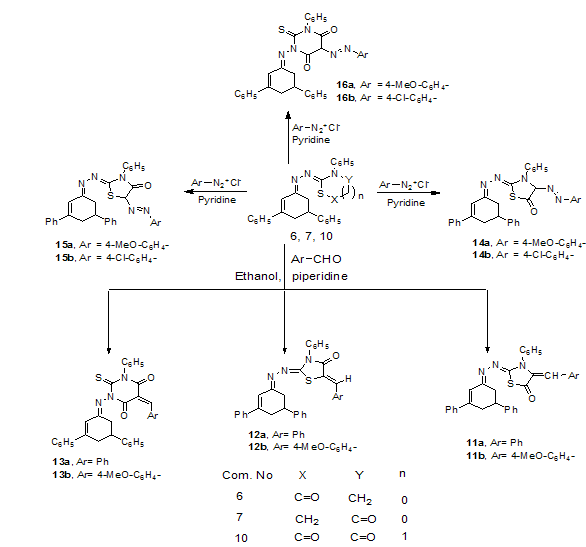 | Scheme 3. |
3. Conclusions
- In conclusion, conducting reactions using thiocarbamoyl derivatives is an effective method for reactions leading to various C—C and C-N bond forming reactions. New thiazolidin-5-ones and 4-ones are of great importance, which were synthesized. The corresponding arylidines and arylazo derivates were also prepared.
4. Experimental
- All melting points were determined on Gallenkamp electric melting point apparatus and were uncorrected. Elemental analyses were carried out at the Microanalytical Unit at Faculty of Science, Mansoura University, Egypt. IR spectra were recorded on a Mattson 5000 Fourier transform infrared (FTIR) spectrometer. The 1H NMR spectra were measured on a Bruker WP 300 in CDCl3 and DMSO-d6 as solvent using tetramethylsilane (TMS) as an internal standard. Mass spectra were recorded on a Finnigan MAT 212 instrument at the Microanalytical Unit at Faculty of Science, Cairo University, Egypt. Compound 1 and 2 were prepared according to a previously reported method [35].3,5-Diphenylcyclohex-2-en-1-one 2. (Method B)Refluxing of 1 (3.2g, 10 mmol) in (20 ml) glacial acetic acid for 6 h. The reaction mixture was poured onto crushed ice then the precipitate was filtered off, dried, and recrystallized from ethanol to give buff powder, mp 82℃ [lit.35 mp 81-83. Method A], yield 95%, IR (KBr) (vmax, cm−1): 1658 (C=O). m/z 248 (M+, 100%).(3,5-Diphenylcyclohex-2-enylidene)hydrazine 3A mixture of 2 (2.48 g, 10 mmol) and hydrazine hydrate (0.6 mL, 12 mmol) in absolute ethanol (15 mL) was refluxed for 3 h. The reaction mixture was left to cool. The precipitated solid was filtered off, dried and recrystallized from ethanol to afford hydrazone derivative 3. Yellow powder, mp 156℃, yield 85%, IR (KBr) (vmax, cm−1): 3353-3316 (NH2), 1614 (C=N). 1H-NMR (CDCl3) δppm = 2.21-2.38 (m, 2H, C4-H), 2.65-2.73 (m, 2H, C6-H), 2.93-3.07 (m, 1H, C5-H), 6.59 (d, 1H, J = 3Hz, C2-H), 7.12-7.57 (m, 10H, Ar-H), 9.46 (s, 2H, NH2). MS: (m/z, %): 262 (100), 245 (54.6), 202 (23.7), 143 (34.2), 115 (36.4), 91 (75.6), 77 (48.05). Anal. Calcd. For C18H18N2 (262.35): C, 82.41, H, 6.92, N, 10.68%. Found: C, 82.53, H, 6.97, N, 10.74%.2-(3,5-Diphenylcyclohex-2-enylidene)-N-phenylhydrazinecarbothioamide 5.To a vigorously stirred solution of 3 (2.62 g, 10 mmol) in dry DMF (10 mL) at room temperature, potassium hydroxide (0.56 g, 10 mmol) and phenylisothiocyanate (1.2 mL, 10 mol) were added simultaneously over 30 min. Stirring was continued for a further 30 min. The reaction mixture was poured into ice cold water and acidified with dilute HCl. The solid obtained was filtered off, dried and recrystallised from ethanol to afford 5. Pale yellow powder, mp 223℃, yield 88%, IR (KBr) (vmax, cm−1): 3192 (2NH), 1614 (C=N). 1H-NMR δppm = 2.31-2.37 (m, 2H, C4-H), 2.65-2.73 (m, 2H, C6-H), 2.83-2.87 (m, 1H, C5-H), 6.59 (d, 1H, J = 3Hz, C2-H), 6.99-7.54 (m, 15H, Ar-H), 8.53, 5.13 (s, 2H, 2NH). MS: (m/z, %): 397 (1.60), 396 (3.70), 395 (9.91), 379 (7.1), 364 (351), 304 (8.1), 262 (100), 245 (70.2), 232 (26.5), 202 (16.5), 143 (34.2), 128 (36.2), 115 (38.7), 91 (75.6), 77 (27.05). Anal. Calcd. for C25H23N3S (397.54): C, 75.53, H, 5.83, N, 10.57%. Found: C, 75.61, H, 5.88, N, 10.61%.2-(3,5-Diphenylcyclohex-2-enylidene)hydrazono)-3-phenylthiazolidin-5-one 6A mixture of compound 5 (3.97 g, 10 mmol), chloroacetyl chloride (0.79 ml, 10 mmol) and potassium hydroxide (0.56 g, 10 mmol) in dry DMF (10 mL) was vigorously stirred at room temperature for 6h. The reaction mixture was poured into ice cold water. The precipitated solid was filtered off, dried and crystallized from ethanol to afford 6. Yellow powder, mp 121℃, yield 85%, IR (KBr) (vmax, cm−1): 1680 (C=O), 1612 (C=N). 1H-NMR δppm =2.78-2.89 (m, 2H, C4-H), 2.97-3.2 (m, 2H, C6-H), 3.46-3.62 (m, 1H, C5-H), 4.1 (s, 2H, CH2 thiazole), 6.58 (d, 1H, J = 3Hz, C2-H), 6.96-7.57 (m, 15H, Ar-H). MS: (m/z, %): 437 (2.25), 408 (2.1), 381 (2.0), 262 (9.5), 212 (7.5), 141 (6.1), 105 (7.2), 93 (100), 77 (16.0). Anal. Calcd. for C27H23N3OS (437.56): C, 74.11, H, 5.30, N, 9.60%. Found: C, 74.16, H, 5.38, N, 9.66%.Thiazolidine derivatives 6, 7, 8a,b and 9To a vigorously stirred solution of hydrazone 3 (2.62 g, 10 mmol) in dry DMF (10 mL) at room temperature, phenylisothiocyanate (1.2 mL, 10 mmol) and potassium hydroxide (0.56 g, 10 mmol) were added simultaneously over 30 min. Stirring was continued for a further 30 min. α-Halo compounds namely, chloroacetyl chloride (0.79 ml, 10 mmol), chloroacetic acid (0.94 g, 10 mmol) or ethyl bromoacetate (1.1 ml, 10 mmol), chloroacetone (0.79 ml, 10 mmol), phenacyl bromide (2.0g, 10 mmol) and chloroacetonitrile (0.63ml, 10 mmol) were added drop wise to the reaction mixture with stirring at 5-10℃ for 3 h then, poured into ice-water, the solid obtained was filtered off, dried and recrystallised from ethanol to afford 6, 7, 8a,b and 9, respectively.2-(3,5-Diphenylcyclohex-2-enylidene)hydrazono)-3-phenylthiazolidin-4-one 7Buff powder, m.p.196℃, yield 70%, IR (KBr) (vmax, cm−1): 1727 (C=O), 1612 (C=N). 1H-NMR δppm =2.79-2.90 (m, 2H, C4-H), 2.98-3.07 (m, 2H, C6-H), 3.17-3.52 (m, 1H, C5-H), 3.99 (s, 2H, CH2 thiazole) 6.51 (d, 1H, J = 3Hz, C2-H), 7.09-7.56 (m, 15H, Ar-H). MS: (m/z, %): 439 (15.0), 438 (28.4), 437 (100), 381 (15.0), 345 (21.2), 288 (26.2), 244 (46.1), 231 (15.8), 215 (15.1), 164 (39.5), 135 (33.2), 91 (95.6), 77 (72.5). Anal. Calcd. for C27H23N3OS (437.56): C, 74.11, H, 5.30, N, 9.60%. Found: C, 74.18, H, 5.33, N, 9.69%.2-(3,5-Diphenylcyclohex-2-enylidene)hydrazono)-4-methyl-3-phenyl-2,3-dihydrothiazole 8aBrown powder, mp 98℃, yield 82%, IR (KBr) (vmax, cm−1): 1609 (C=N). 1H-NMR δppm =1.87(s, 3H, CH3), 2.33-2.43 (m, 2H, C4-H), 2.71-2.82 (m, 2H, C6-H), 2.91-2.96 (m, 1H, C5-H), 3.02, 3.11 (d.d, 2H, CH2 thiazole), 3.07-3.14 (m, 1H, CH thiazole), 6.76 (d, 1H, J = 3Hz, C2-H), 7.12-7.57 (m, 15H, Ar-H). MS: (m/z, %):436 (10.8), 435 (36.1), 304 (19.4), 266 (12.8), 189 (12.8), 176 (14.7), 141 (13.1), 135 (22.1), 118 (20.2), 104 (12.0), 93 (100), 77 (37.4). Anal. Calcd. For C28H25N3S (435.58): C, 77.21; H, 5.79; N, 9.65%. Found: C, 77.18; H, 5.75; N, 9.63%.2-(3,5-diphenylcyclohex-2-enylidene)hydrazono)-3,4-diphenyl-2,3-dihydrothiazole 8bReddish brown powder, mp 104℃, yield 85%, IR (KBr) (vmax, cm−1): 1608 (C=N). 1H-NMR δppm = 2.35-2.44 (m, 2H, C4-H), 2.73-2.82 (m, 2H, C6-H), 2.94-3.06 (m, 1H, C5-H), 3.21, 3.25 (d.d, 2H, CH2 thiazole), 4.06-4.12 (m, 1H, CH thiazole), 6.62 (d, 1H, J = 3Hz, C2-H), 6.98-7.63 (m, 20H, Ar-H), MS: (m/z, %): 497 (32.6), 486 (8.9), 456 (22.7), 437 (4.0), 406 (3.1), 381 (4.9), 304 (17.1), 262 (21.4), 247 (12.1), 143 (21.5), 118 (18.9), 93 (100), 77 (24.1). Anal. Calcd. For C33H27N3S (497.65): C, 79.64, H, 5.47, N, 8.44%. Found: C, 79.75, H, 5.38, N, 8.48%.2-(3,5-Diphenylcyclohex-2-enylidene)hydrazono)-3-phenylthiazolidin-4-imine 9Buff powder, mp 122℃, yield 80%, IR (KBr) (vmax, cm−1): 3346 (NH), 1604 (C=N).1H-NMR δppm =2.31-2.38 (m, 2H, C4-H), 2.65-73 (m, 2H, C6-H), 2.83-2.87(m, 1H, C5-H), 3.96 (s, 2H, CH2 thiazole), 6.42 (d, 1H, J = 3Hz, C2-H), 7.07-7.58 (m, 15H, Ar-H), 8.03 (s, 1H, NH). MS: (m/z, %): 436 (10.8), 435 (36.1), 304 (19.4), 266 (12.8), 189 (12.8), 176 (14.7), 141 (13.1), 135 (22.1), 118 (20.2), 104 (12.0), 93 (100), 77 (37.4). Anal. Calcd. For C27H24N4S (436.57): C, 74.28, H, 5.54, N, 12.83%. Found: C, 74.24, H, 5.48, N, 12.85%.1-(3,5-Diphenylcyclohex-2-enylidene)amino)-3-phenyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 10To a stirred solution of 5 (5.96 g, 0.015 mol) in acetyl chloride (10 mL), malonic acid (2.1g, 0.02mol) was added. The reaction mixture was left for 2 h at ambient temperature and then heated for 4 hrs at 50–55℃. The contents were then poured onto crushed ice, cooled to 10℃, and the separated solid was filtered off, dried and recrystallized from the ethanol to give the corresponding thiobarbituric acid derivatives 10. Brown crystals, mp 136℃, yield 74%, IR (KBr) (vmax, cm−1): 1685,1652 (2C=O), 1604 (C=N). 1H-NMR δppm =2.29-2.45 (m, 2H, C4-H), 2.76-2.89 (m, 2H, C6-H), 2.98-3.04 (m, 1H, C5-H), 3.21 (s, 2H, CH2), 6.17 (d, 1H, J = 3Hz, C2-H), 6.71-7.26 (m, 15H, Ar-H). MS: (m/z, %): 465 (1.37), 458 (4.6), 437 (2.56), 420 (2.42), 402(11.9), 377 (5.1), 361 (5.65), 347 (4.32), 321 (9.2), 302 (17.7), 287 (46.2), 262 (21.0), 245 (100), 230 (99), 205 (24.7) 202 (24.0), 174 (9.9), 152 (12.7), 119 (34.1), 91 (52.5), 77 (56.6). Anal. Calcd. For C28H23N3O2S (465.57): C, 72.23, H, 4.98, N, 9.03%. Found: C, 72.31, H, 5.06, N, 9.13%.Synthesis of arylidene derivatives 11a,b, 12a,b, and 13a,bTo a solution of 6, 7, or 10 (0.01 mole and aromatic aldehydes namely, benzaldehyde (1.06 g, 0.01 mol) or p-methoxybenzaldehyde (1.36 g, 0.01 mol) in ethanol (20 mL), was added a few drops of piperidine and the reaction mixture was refluxed for 4h then left to cool. The precipitate that formed was filtered off, washed with ethanol and recrystallized from ethanol to afford the corresponding derivatives 11a, b, 12a, b and 13a, b, respectively.4-Benzylidene-2-((-(3,5-diphenylcyclohex-2-enylidene) hydrazono)-3-phenyl thiazolidin-5-one 11aBuff powder, mp 241℃, yield 74%, IR (KBr) (vmax, cm−1): 1687 (C=O), 1610 (C=N). 1H-NMR δppm = 2.31- 2.37(m, 2H, C4-H), 2.65-73(m, 2H, C6-H), 2.83-2.87(m, 1H, C5-H), 6.29 (d, 1H, J = 3Hz, C2-H), 6.81 (s, 1H, olefinic CH), 6.99-7.54 (m, 20H, Ar-H). MS: (m/z, %): 525 (7.2), 449 (19.1), 435 (9.2), 345 (22.4), 304 (11.6), 285 (32.5), 260 (40.9), 245 (93.4), 143 (43.6), 91 (100), 77 (56.5). Anal. Calcd. For C34H27N3OS (525.66): C, 77.69, H, 5.18, N, 7.99%. Found: C, 77.74, H, 5.25, N, 8.06%.2-((3,5-Diphenylcyclohex-2-enylidene)hydrazono)-4-(4-methoxy benzylidene)-3-phenylthiazolidin-5-one 11bWhite powder, mp 263℃, yield 79%, IR (KBr) (vmax, cm−1): 1684 (C=O), 1603 (C=N). 1H-NMR δppm = 2.31- 2.37(m, 2H, C4-H), 2.65-73(m, 2H, C6-H) , 2.83-2.87(m, 1H, C5-H), 3.84 (s, 3H, OCH3), 6.29 (d, 1H, J = 3Hz, C2-H), 6.81 (s, 1H, olefinic CH), 6.99-7.54 (m, 19H, Ar-H). Anal. Calcd. For C35H29N3O2S (555.69): C, 75.65, H, 5.26, N, 7.56%. Found: C, 75.71, H, 5.34, N, 7.63%.(2Z,5Z)-5-Benzylidene-2-((E)-(3,5-diphenylcyclohex-2-enylidene) hydrazono)-3-phenyl thiazolidin-4-one 12aPale yellow powder, mp 183℃, yield 69%, IR (KBr) (vmax, cm−1): 1687 (C=O), 1605 (C=N). 1H-NMR δppm = 2.31-2.37(m, 2H, C4-H), 2.65-73(m, 2H, C6-H), 2.83-2.87(m, 1H, C5-H), 6.23 (d, 1H, J = 3Hz, C2-H), 7.03-7.54 (m, 20H, Ar-H), 7.78 (s, 1H, olefinic CH). Anal. Calcd. For C34H27N3OS (525.66): C, 77.69, H, 5.18, N, 7.99%. Found: C, 77.76, H, 5.26, N, 8.03%.(2Z,5Z)-2-((E)-(3,5-Diphenylcyclohex-2-enylidene)hydrazono)-5-(4-methoxybenzylidene)-3-phenylthiazolidin- 4- one 12bPale yellow powder, mp 206℃, yield 71%, IR (KBr) (vmax, cm−1): 1681 (C=O), 1604 (C=N). MS: (m/z, %): 555 (4.3), 524 (12.7), 449 (6.5), 435 (26.8), 381 (12.4), 345 (28.6), 288 (32.7), 262 (56.1), 244 (39.1), 231 (25.4), 164 (41.3), 135 (28.5), 91 (100), 77 (76.4). Anal. Calcd. For C35H29N3O2S (555.69): C, 75.65, H, 5.26, N, 7.56%. Found: C, 75.70, H, 5.28, N, 7.64%.5-Benzylidene-1-(3,5-diphenylcyclohex-2-enylideneamino)-3-phenyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 13aYellow crystal, mp 174℃, yield 58%, IR (KBr) (vmax, cm−1): 1686,1653 (2C=O), 1602 (C=N). MS: (m/z, %): 553 (12.7), 525 (3.9),476 (4.3),463 (6.2), 458 (4.6), 437 (2.56), 420 (2.42), 402(11.9), 377 (5.1), 361 (5.65), 347 (4.32), 321 (9.2), 302 (17.7), 287 (46.2), 262 (21.0), 245 (100), 230 (99), 215 (24.7) 202 (24.0), 174 (9.9), 152 (12.7), 119 (34.1), 91 (52.5), 77 (56.6). Anal. Calcd. For C35H27N3O2S (553.67): C, 75.92, H, 4.92, N, 7.59%. Found: C, 75.94, H, 4.93, N, 7.61%.1-(3,5-Diphenylcyclohex-2-enylideneamino)-5-(4-methoxybenzylidene)-3-phenyl-2-thioxodihydropyrimidine- 4,6(1H,5H)-dione 13bYellow powder, mp 216℃, yield 84%, IR (KBr) (vmax, cm−1): 1684, 1651 (2C=O), 1602 (C=N). 1H-NMR δppm = 2.32-2.39(m, 2H, C4-H), 2.65-73(m, 2H, C6-H), 2.83-2.87(m, 1H, C5-H), 3.90 (s,3H,OCH3), 6.22 (d, 1H, J = 3Hz, C2-H), 7.02-7.56 (m, 19H, Ar-H), 8.21 (s, 1H, olefinic CH). Anal. Calcd. For C36H29N3O3S (583.70): C, 74.08, H, 5.01, N, 7.20%. Found: C, 74.12, H, 5.03, N, 7.21%.Synthesis of arylazo derivatives 14a,b, 15a,b and 16a,bA solution of sodium nitrite (0.70 g in 10 mL water) was gradually added to a well cooled (0-5℃) solution of the aromatic amines namely; p-anisidine, p-chloroaniline (0.01 mmol) in concentrated HCl (3.0 mL). The diazonium salt solution was added with continuous stirring to a cold (0-5℃) solution of compounds 6, 7 or 10 (0.01 mol) in pyridine (30 mL). The reaction mixture was allowed to stir at (0–5℃) for 2 hrs, and then the solid was collected by filtration. The crude products thus obtained, were dried and recrystallized from ethanol to give the corresponding arylazo derivatives 14a, b, 15a, b and 16a, b, respectively.2-(3,5-Diphenylcyclohex-2-enylidene)hydrazono)-4-(4-methoxyphenyl) diazenyl)-3-phenylthiazolidin-5-one 14aYellow powder, mp 106℃, yield 64%, IR (KBr) (vmax, cm−1): 1683 (C=O), 1605 (C=N). 1H-NMR δppm = 2.31-2.37(m, 2H, C4-H), 2.65-73(m, 2H, C6-H), 2.83-2.87(m, 1H, C5-H), 3.51 (s, 1H,CH thiazolidine), 3.82 (s,3H,OCH3), 6.64 (d, 1H, J = 3Hz, C2-H), 6.95-7.59 (m, 19H, Ar-H). MS: (m/z, %): 571 (4.1), 543 (0.4), 522 (0.7), 509 (0.8), 492 (1.49), 456 (2.29), 437 (37), 345 (9.5), 304 (34.5), 262 (31.3), 254 (50), 115 (45.1), 91 (100), 77 (48.4). Anal. Calcd. For C34H29N5O2S (571.69): C, 71.43, H, 5.11, N, 12.25%. Found: C, 71.52, H, 5.18, N, 12.33%.4-(4-Chlorophenyl)diazenyl)-2-(3,5-diphenylcyclohex-2-enylidene) hydrazono)-3-phenylthiazolidin-5-one 14bRed powder, mp 109℃, yield 72%, IR (KBr) (vmax, cm−1): 1682 (C=O), 1606 (C=N). 1H-NMR δppm = 2.31-2.37(m, 2H, C4-H), 2.65-73(m, 2H, C6-H), 2.83-2.87(m, 1H, C5-H), 3.54 (s, 1H, CH thiazolidine), 6.55 (d, 1H, J = 3Hz, C2-H), 6.99-7.68 (m, 19H, Ar-H). MS: (m/z, %): 578 (2.18), 576 (3.61), 550 (2.85), 522 (1.1), 492 (18.3), 435 (9.2), 350 (13.2), 304 (15.2), 285 (14.1), 262 (40.9), 245 (93.4), 143 (43.6), 103 (26.3), 91 (100), 77 (56.5). Anal. Calcd. For C33H26ClN5OS (576.11): C, 68.80, H, 4.55, N, 12.16%. Found: C, 68.83, H, 4.61, N, 12.26%.2-(3,5-Diphenylcyclohex-2-enylidene)hydrazono)-5-(4-methoxyphenyl) diazenyl)-3-phenylthiazolidin-4-one 15aRed powder, mp 121℃, yield 56%, IR (KBr) (vmax, cm−1): 1684 (C=O), 1608 (C=N). MS: (m/z, %): 571 (8.4), 522 (4.3), 509 (2.1), 492 (6.5), 435 (26.8), 381 (12.4), 345 (28.6), 288 (32.7), 262 (56.1), 244 (39.1), 231 (25.4), 164 (41.3), 135 (28.5), 91 (100), 77 (76.4). Anal. Calcd. For C34H29N5O2S (571.69): C, 71.43, H, 5.11, N, 12.25%. Found: C, 71.37, H, 5.05, N, 12.28%.5-(4-Chlorophenyl)diazenyl)-2-(3,5-diphenylcyclohex-2-enylidene) hydrazono)-3-phenylthiazolidin-4-one 15bRed powder, mp 121℃, yield 60%, IR (KBr) (vmax, cm−1): 1685 (C=O), 1604 (C=N). 1H-NMR δppm =2.31-2.37(m, 2H, C4-H), 2.65-73(m, 2H, C6-H), 2.83-2.87(m, 1H, C5-H), 3.51 (s, 1H, CH thiazole), 6.52 (d, 1H, J= 3Hz, C2-H), 6.99-7.68 (m, 19H, Ar-H). MS: (m/z, %): 576 (61.3), 550 (37.1), 492 (43.6), 436 (100), 350 (22.3), 305 (12.7), 285 (21.4), 262 (39.6), 245 (76.9), 143 (54.5), 91 (91.3), 77 (43.1). Anal. Calcd. For C33H26ClN5OS (576.11): C, 68.80, H, 4.55, N, 12.16%. Found: C, 68.83, H, 4.61, N, 12.26%.1 - (3 , 5 - Diphenylcyclohex - 2 - enylideneamino ) - 5 - ( ( 4-methoxyphenyl)diazenyl)-3-phenyl-2–thioxodihydro pyrimidine – 4 , 6-( 1H,5H)-dione 16aYellow powder, mp 121℃, yield 63%, IR (KBr) (vmax, cm−1): 1684,1653 (2C=O), 1604 (C=N). 1H-NMR δppm = 2.29-2.45 (m, 2H, C4-H), 2.76-2.89 (m, 2H, C6-H), 2.98-3.04 (m, 1H, C5-H), 3.41 (s, 1H, CH), 3.82 (s,3H,OCH3), 6.17 (d, 1H, J = 4Hz, C2-H), 6.71-7.26 (m, 19H, Ph-H). Anal. Calcd. For C35H29N5O3S (599.70): C, 70.10, H, 4.87, N, 11.68%. Found: C, 70.08, H, 4.85, N, 11.66%.5-(4-Chlorophenyl)diazenyl)-1-(3,5-diphenylcyclohex-2-enylidene-amino)-3-phenyl-2-thioxodihydropyrimidine-4,6-(1H,5H)-dione 16bYellow powder, mp 121℃, yield 69%, IR (KBr) (vmax, cm−1): 1682,1654 (2C=O), 1604 (C=N). 1H-NMR δppm = 2.28-2.47 (m, 2H, C4-H), 2.79-2.91 (m, 2H, C6-H), 2.99-3.05 (m, 1H, C5-H), 3.44 (s, 1H, CH), 6.17 (d, 1H, J = 4Hz, C2-H), 6.86-7.54 (m, 19H, Ar-H). Anal. Calcd. For C34H26ClN5O2S (604.12): C, 67.60, H, 4.34, N, 11.59 Found: C, 67.62, H, 4.36, N, 11.61%.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML