| [1] | R. Datta, Hydroxy carboxylic acids, in Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed, Seidel A.Wiley, Ed., Hoboken, NJ, Vol. 14, pp.114-134, 2005 |
| [2] | Soomro, A.H., Masud. T, and Anwaar, K., 2002, Role of lactic acid bacteria (LAB) in food preservation and human health – A Review., Pakistan J. Nutrition, 1(1), 20-24 |
| [3] | Mills, C.A. Navarro, M., Engel, E., Martinez, E. Ginebra, M.P., Planell, J., Abdelhamid Errachid, A., and Samitier, J., 2006, Transparent micro- and nano-patterned poly(lactic acid) for biomedical applications., J Biomed. Mater. Res. A., 76(4), 781-787 |
| [4] | Avinc, O., and Khoddami, A., 2009, Overview of poly(lactic acid) (PLA) Fibre Part I: Production, properties, performance, environmental impact, and end use applications of poly(lactic acid) fibres., Fibre Chem., 41(6), 50-56 |
| [5] | Lipinsky, E.S., and Sinclair, R.G., 1986, Is lactic acid a commodity chemical?, Chem. Engg. Prog,,82, 26-32 |
| [6] | Datta, R, Tsai S.P., Bonsignore, P., Moon, S.H., and Frank, J.R., 1995, Technological and economic potential of poly(lactic acid) and lactic acid derivatives., FEMS Microbiol. Rev., 16, 221-231 |
| [7] | Yadav, A.K., Chaudhari, A.B., and Kothari, R.M., 2011, Bioconversion of renewable resources into lactic acid: an industrial view., Crit. Rev. Biotechnol., 31(1), 1-19 |
| [8] | Jiao, Y.-H., Li, Y., Wang, S., Zhang, K., Jia, Y.-G., and Fu, Y., 2010, Layer by Layer assembly of poly(lactic acid) nanoparticles: A facile way to fabricate films for model drug delivery., Langmuir: The ACS Journal of Surfaces And Colloids, 26(11), 8270-8273 |
| [9] | J. David, “Drinking water criteria document for chloramines,” Medical University of South Carolina, Health and Ecological Criteria Division, 1994.(http://www.epa.gov/ncea/pdfs/water/chloramine/dwchloramine.pdf) |
| [10] | Singh B., Singh A.K., Singh N.B., and Saxena, B.B.L.,1984, Ru(III) catalysis in oxidation of n-propanol and n-butanol by acidic solutions of bromamine-ULE., Tetrahedron. 40, 5203-5206 |
| [11] | Trieff, N.M., Made Gowda, N .M., and Sadagopa Ramanujam, V.M., 1980, Chloramine-T as a potential scrubbing agent: Removal of odorous sulfur-containing environmental pollutants., Bull. Environm. Contam. Toxicol, 24, 383-388 |
| [12] | Veeraiah, M.K., Ananda Murthy, A.S., and Made Gowda, N.M., 2010, Oxidation of n-propylamine and n-butylamine by chloramines-B and chloramine-T in basic medium: A kinetic study., Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 40(4), 225-230 |
| [13] | Ramachandrappa, R., Puttaswamy, Mayanna, S.M., and Made Gowda, N.M., 1999, Kinetics and mechanism of oxidation of aspirin by bromamine-T, N-bromosuccinimide and N-bromophthalimide., Int. J. Chem. Kinet., 30(6), 407-414 |
| [14] | Venkatesha, B.M., Ananda, S., Mahadevappa, D.S., and Made Gowda, N.M., 1995, Kinetic and mechanistic studies of indigocarmine oxidation by chloramines-T and chlorine in acidic buffer media., Int. J. Chem. Kinet., 27(7), 663-674 |
| [15] | Khan, K., Raju, M.K., and Ud Din, K., 2004, Oxidation of lactic acid by water soluble (colloidal) manganese dioxide., Int. J. Chem. Kinet., 36: 359-366 |
| [16] | Wang, H.-Y., and Zhao, J.-F., 2003, Interaction of indigo carmine with cetyltrimethylammonium bromide and application to determination of cationic surfactant in waste water., Bull. Korean Chem. Soc., 24(10), 1444-1448 |
| [17] | Farah, M.E., Maia, M., and Rodrigues, E.B., 2009, Dyes in ocular surgery: principles for use in chromovitrectomy., Amer. J. Ophthalmol., 148, 332-340 |
| [18] | Hunt, I.V., 1939, The indigo carmine reaction as a test for chlorates and hypochlorites in milk., Analyst, 64, 653-657 |
| [19] | Dweck, A.C., 2002, Natural ingredients for colouring and styling., Int. J. Cosmet. Sci., 24(5), 287-302 |
| [20] | Komboonchoo, S., and Bechtold, T., 2009, Natural dyeing of wool and hair with indigo carmine (C.L. Natural Blue 2), a renewable resource based blue dye., J. Cleaner Production, 17, 1487-1493 |
| [21] | E.H. Rodd, Chemistry of carbon compounds., Elsevier, Amsterdam,IVB: p.1093, 1960 |
| [22] | Keyvanfard, M., 2009, Kinetic-photometric determination of iodide based on its inhibitory effect on the bromated oxidation of indigo carmine in micellar medium., Asian J. Chem., 21(4), 2715-2720 |
| [23] | Zaprozhets, O.A., and Biokon, S.L., 2007, A visual test method for determining selenium(IV) with indigo carmine immobilized on silica., J. Anal. Chem., 62, 188-189. |
| [24] | Cholkar, K., Kouassi, G.K., Ananda, S., Veeraiah, M.K., and Made Gowda, N.M., 2011, Osmium(VIII)-catalyzed kinetics and mechanism of indigo carmine oxidation by chloramine-B in basic Medium., Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 41(9), 1126-1134 |
| [25] | E.A. Moelwyn-Hughes, Ed., Kinetics of reactions in solutions., Oxford University Press, London, pp. 297-299, 1947 |
| [26] | Campbell, M.M., and Johnson, G., 1978, Chloramine-T and related N-halogeno-N-metallo reagents., Chem. Rev., 78, 65-79 |
| [27] | Mallamma, Ananda, S., Rangaswamy, and Made Gowda, N.M., 2003, Kinetic and mechanistic studies of Pd(II) catalyzed oxidation of some α-hydroxy acids by sodium N-bromobenzenesulphonamide in hydrochloric acid solutions., Synth. React. Inorg. Met.-Org. Chem., 33, 1555-1568 |
| [28] | Sushashini, M., Subramanian, M., and Rao,V.R.S., 1985, Determination of protonation constant of chormaine-B., Talanta, 32, 1082-1085 |
| [29] | Hardy, F.F., and Johnston, J.P., 1973, J. Chem. Soc., Perkin Trans. II, 742 |
| [30] | Narayanan, S.S., and Rao, V.R.S, 1983, Chlorine isotopic exchange reaction between chloramine-T and chloride ion., Radiochim. Acta, 32(4), 211-214 |
| [31] | K.J. Laidler, Reaction Kinetics: 2nd Ed., Tata Mc-Graw Hill, New Delhi, 1995 |

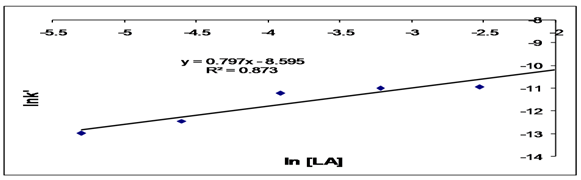
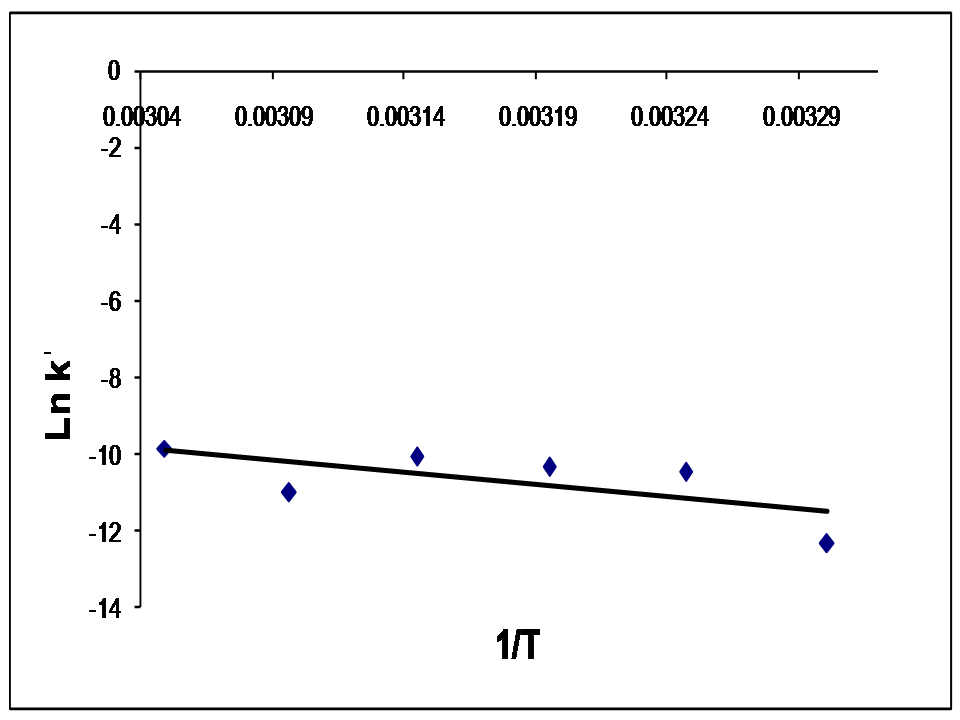
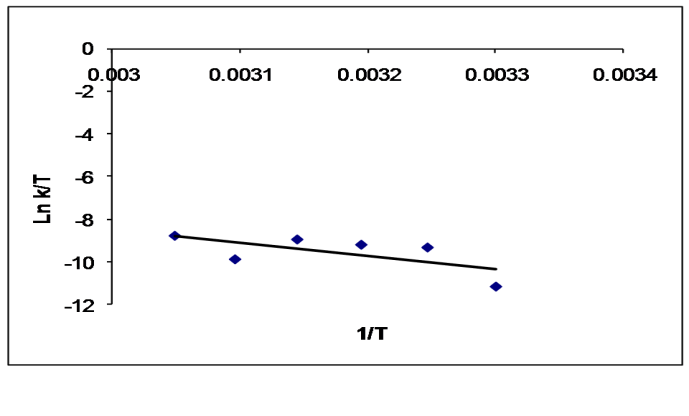
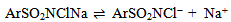
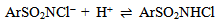
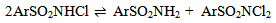
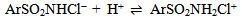
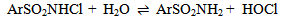
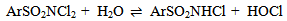
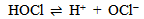
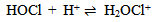
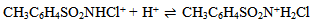
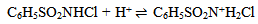
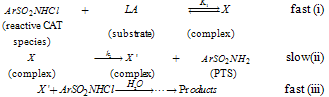
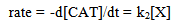
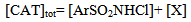
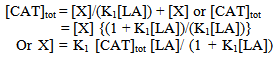
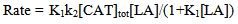
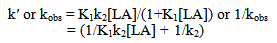
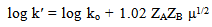
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML