-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(1): 14-17
doi:10.5923/j.ajoc.20120201.03
Efficient Method for the Synthesis of Diazaphospholidines: Toxicological Evaluation
Fouzia Bouchareb1, Sihem Hessainia1, Malika Berredjem1, Hounaida Benbouzid2, Houria Djebbar1, Nour-Eddine Aouf1
1Laboratory of Applied Organic Chemistry, Badji-Mokhtar University BP12. Annaba, 23000, Algeria
2Laboratory of Cell Toxicology, General Direction of Scientific Research and Technological Development, Algeria
Correspondence to: Malika Berredjem, Laboratory of Applied Organic Chemistry, Badji-Mokhtar University BP12. Annaba, 23000, Algeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this work, we described the synthesis of new heterocyclic organophosphorus compounds starting from a primary amine and phenyl phosphonic dichloride (PDCP). The introduction of a phosphoryl group into heterocyclic structures can generate potential chemical and biological activities. We have prepared the phosphoramidates in only one step. These compounds provide access to diazaphospholidine by intermolecular cyclization using dibromoethane. We chose primary amines (cyclohexylamine, benzylamine, propylamine and phenyl ethylamine) to prepare phosphoramidates. The toxicological activity of two synthesized molecules was evaluated.
Keywords: Phenyl Phosphonic Dichloride, Phosphoramidate, Diazaphospholidine
Cite this paper: Fouzia Bouchareb, Sihem Hessainia, Malika Berredjem, Hounaida Benbouzid, Houria Djebbar, Nour-Eddine Aouf, Efficient Method for the Synthesis of Diazaphospholidines: Toxicological Evaluation, American Journal of Organic Chemistry, Vol. 2 No. 1, 2012, pp. 14-17. doi: 10.5923/j.ajoc.20120201.03.
Article Outline
1. Introduction
- Organophosphorus compounds are ubiquitous in nature and find applications in the field of agriculture, medicine and industry.1,2 Some organophosphorus compounds have been described in the literature as inhibitors of bacterial3, herbicides, insecticides, pesticides4, antifungal agents5, anti-HIV6, anti-cancer7, antiviral andanti-inflammatory8. An important group of this class is phosphoramidate, which have been used in many reactions and synthesis of organic compounds.A number of research groups has become interested in organophosphorus heterocyclic compounds since they are finding extensive use as pesticides in agriculture, as stabilizers in polymers and as lubricant oil additives. An important family of this heterocycles is diazaphospholidine, which have been used in many reactions serve as ligands for transition metal complexes9 phosphorus ligands containing alternative donor units, such as phosphites10 or diazaphospholidines,11 have been less widely examined.Herein, we report the synthesis of some novel diazaphospholidine derivatives (2a-d), and their corresponding precursors phosphoramidates (1a-d). choose to use phenylphosphonic dichloride to introduce phosphoramidate moiety. The toxicological activity of this compounds was evaluated.
2. Results and discussion
- The phosphoramidates derivatives (1a-d), (Scheme 1) were prepared in a one-pot synthetic route, starting from corresponding primary amines with phenyl phosphonic dichloride in dry acetonitrile at -5°C. These compounds were obtained in good yields. The synthesis of diazaphosph-olidine was achieved by mixing 10 equivalents of dibromoetane with phosphoramidate in the presence of potassium carbonate. The heterocyclic compounds were obtained as a white solid in 67 % yields.
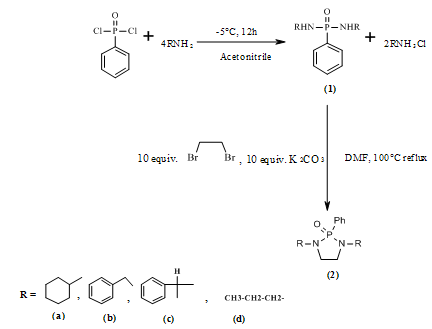 | Scheme 1. |
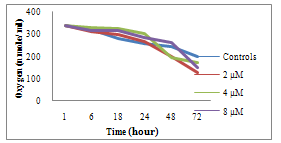 | Figure 1. Effects of the molecule (1a) on the respiratory metabolism |
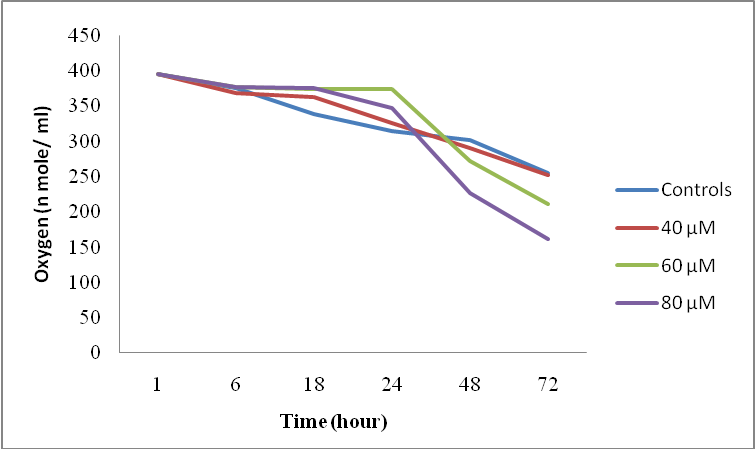 | Figure 2. Effects of the molecule (1b) on the respiratory metabolism |
3. Experimental Section
- Melting points were determined in open capillary tubes on an Electro thermal apparatus and uncorrected. IR spectra were recorded on a perkin-Elmer FT-600 spectrometer. Proton nuclearmagnetic resonance was determined with a 360 WB or AC 250-MHz Bruker spectrometer using CDCl3 and DMSO-d6 as a solvent and TMS as an internal standard. Chemical shifts are reported in δ units (ppm). All coupling constants (J ) are reported in Hertz. Multiplicity is indicated as s (singlet), d (doublet), t (triplet), m (multiplet) and combination of these signals. Electron ionization mass spectra (30 ev) were recorded in positive mode on a Water MicroMass ZQ. Hight-resolution mass were measured on a Joel SX 102 mass spectrometer and recorded in FAB positive mode. All reactions were monitored by TLC on silica Merck h60 F254 (Art. 5554) precoated alumium plates and were developed by spraying with ninhydrin solution.
3.1. Synthesis of Phenyl Phosphoramidates
- To a stirred solution of phenyl phosphonic dichloride (0.71 mL, 5 mmol) in dry acetonitrile (35 mL), a solution of primary amine (1.98 g, 20 mmol, 4 equiv) was added dropwise at -5°C. After 12 h, the solvent was evaporated under vacuum and the oily product was washed with distilled water. The organic layer was dried over sodium sulfate and concentrated. The products were purified by column chromatography on silica gel (CH2Cl2/MeOH 9/1). The phosphoramidates were obtained as a white solid in excellent yields.Bis (cyclohexylamino) phenylphosphine oxide 1a: Yield: 89%. M = 320 g /mol [C18H29N2OP]. Mp. 156-158 °C. Rf =0.625 (CH2Cl2/MeOH). IR (KBr, cm-1): 3155.3 cm-1 (NH), 1461.9 cm-1 (C=CAr), 1180.4 cm-1 (P=O), 1110.9 cm-1 (C-N-P). 1H NMR (CDCl3, 250MHz): 7.90 (m, 2H, H-Ar); 7.50 (m, 3H, H-Ar); 3.15 (m, 2H, CH-cyc); 2.40 (d, J= 9.3 Hz, 2H, NH-P=O); 1.90 (m, 4H, CH2-cyc); 1.65 (m, 4H, CH2-cyc); 1.15 (m, 12H, CH2 cycl). 31P NMR (CDCl3, 200.7MHz) δ = 16.42 ppm. 13C NMR (CDCl3, 250MHz): 134 (CH-Ar); 132 (CH-Ar); 129 (CH-Ar); 55 (CH-Hex); 35(CH2-Hex); 25 (CH2- Hex); 24.8 (CH2- Hex).Bis (benzylamino) phenylphosphine oxide 1b: Yield: 91%. M = 336 g /mol [C20H21N2OP]. Mp. 159-161°C. Rf = 0.60 (CH2Cl2/MeOH). IR (KBr, cm-1): 3163cm-1 (NH),1461.9 cm-1 (C=CAr), 1253.6 cm-1 (P=O), 1118.6 cm-1 (C-N-P). 1H NMR (CDCl3, 250MHz): 7.9 (m, 2H, H-Ar); 7.5 (m, 3H, H-Ar); 7.25 (m, 10H, H-Ar); 4.15 (d, J= 6.85 Hz, 4H, CH2-N); 3.0 (m, 2H, NH-P=O). 31P NMR (CDCl3, 200.7MHz) δ = 16.33 ppm. 13C NMR (CDCl3, 250MHz): 141 (C-Ar); 134.1 (C-Ar); 134 (CH-Ar); 132 (CH-Ar); 129 (CH-Ar); 128.5 (CH-Ar); 127 (CH-Ar); 126.6 (CH-Ar); 44 (CH2).Bis (phenylethylamino) phenylphosphine oxide 1c: Yield: 87.15%. M= 364 g /mol [C22H25N2OP]. Mp. 164-167 °C. Rf= 0.63 (CH2Cl2/MeOH).IR (KBr, cm1) : 3209.3 cm-1 (NH), 1450.4cm-1 (C=C Ar ), 1191.9 cm-1 (P=O), 1029.9 cm-1 (C-N-P). 1H NMR (CDCl3, 250MHz): 7.75 (m, 2H, H-Ar); 7.4 (m, 3H, H-Ar); 7.35-7 (m, 10H, H-Ar); 4.60 (m, 1H,*CH-NH); 4.35 (m, 1H,*CH-NH); 2.75 (s, 2H, NH-P=O); 1.5 (d, J = 6.25 Hz, 3H, CH3); 1.3 (d, J= 6.77 Hz, 3H, CH3). 31P NMR (CDCl3, 200.7MHz) δ = 16.65 ppm. 13C NMR (CDCl3, 250MHz): 145 (C-Ar); 134.2 (C-Ar); 132.1 (CH-Ar); 130 (2 C, CH-Ar); 128.8 (CH-Ar); 128.5 (CH-Ar); 126.9 (CH-Ar); 126.7 (CH-Ar); 55 (CH); 24 (CH3). Bis (propylamino) phenylphosphine oxide 1d: Yield: 88.53%. M = 240 g /mol [C12H21N2OP]. Mp. 150-153 °C. Rf = 0.617 (CH2Cl2/MeOH). IR (KBr, cm-1): 3193.9 cm-1 (NH), 1465.8 cm-1 (C=C Ar), 1200 cm-1 (P=O), 1033.8 cm-1 (C-N-P). 1H NMR (CDCl3, 250MHz): 7.9 (m, 2H, H-Ar); 7.5 (m, 3H, H-Ar); 2.9 (m, 4H, CH2 -NH); 2.5 (m, 2H, NH-P=O); 1.5 (m, 4H, CH2-CH3); 0.9 (t, J1= 7.31 Hz, J2= 7.46 Hz, 6H, CH3). 31P NMR (CDCl3, 200.7MHz) δ = 16.4 ppm. 13C NMR (CDCl3, 250MHz): 135 (C-Ar); 131.5 (CH-Ar); 128.3 ( CH-Ar); 128.30 (CH-Ar); 42.5(CH2-NH); 25.3 ( CH2- CH3); 11.3 (CH3).
3.2. Synthesis of Diazaphospholidine
- A solution of phenyl phosphoramidates (1 g, 3 mmol, 1 equiv) and excess of K2CO3 (4.14 g, 30 mmol, 10 equiv) in DMF (2 mL) was stirred, a solution of 1, 2- dibromoethane (2.59 g, 30 mmol, 10 equiv) was added dropwise. The reaction mixture was refluxed for 2h. The residue was evaporated in vacuum. The products were purified by column chromatography on silica gel (CH2Cl2/MeOH 9/1) afforded diazaphospholidines as a white solid in 67 % yields.1,3-cyclohexyl, 2-phenyl diazaphospholidin-2-oxide 2a: Yield: 66.78%. M =346 g /mol [C20H31N2OP]. Mp. 171-173°C. Rf = 0.83 (CH2Cl2/MeOH). IR (KBr, cm-1): 1451.5 cm-1(C=C Ar ), 1181.2 cm-1 (P=O), 1109.4 cm-1 (C-N-P). 1H NMR (CDCl3, 250MHz): 7.95 (m, 2H, H-Ar); 7.55 (m, 3H, H-Ar); 3.20 (m, 2H, CH-cyc); 2.5 (t, J1= 9.4 Hz, J2= 9.42 Hz, 4H, CH2-cyc à 5); 1.95 (m, 4H, CH2-cyc);1.70 (m, 4H, CH2-cyc); 1.20 (m, 12H, CH2- cycl). 31P NMR (CDCl3, 200.7MHz)δ = 33.55 ppm. 13C NMR (CDCl3, 250MHz): 134.2 (C-Ar); 131.9 (1 C, CH-Ar); 130.3 (CH-Ar); 128.8 (CH-Ar); 60 (CH-Hex); 44.1 (CH2-cyc); 33.1 (CH2- Hex); 25.7 (CH2- Hex); 25.1 (CH2- Hex).1, 3-benzyl, 2-phenyl diazaphospholidin-2-oxide 2b: Yield: 69.42%. M = 346g/mol [C22H31N2OP]. Mp. 174-177 °C. Rf = 0.81 (CH2Cl2/MeOH). IR (KBr, cm-1): 1460.1 cm-1 (C=CAr),1253.4 cm-1(P=O), 1117.6 cm-1 (C-N-P). 1H NMR (CDCl3, 250MHz): 7.95 (m, 2H, H-Ar); 7.55 (m, 3H, H-Ar); 7.30 (m, 10H, H-Ar); 4.20 (d, J= 6.95 Hz, 4H, CH2-N);3.0 (t, J1= 8.8 Hz, J2= 8.9 Hz,4H, CH2cyc à 5). 31P NMR (CDCl3, 200.7MHz) δ = 33.34 ppm. 13C NMR (CDCl3, 250MHz): 136.4 (C-Ar); 134.2 (C-Ar); 134.1 (CH-Ar); 132.3 (CH- Ar); 128.8 (CH-Ar); 128.5 (CH-Ar); 127.9 (CH-Ar); 127(CH-Ar); 73.3 (CH2); 45.6 (CH2).1, 3-phenylethyl, 2-phenyl, diazaphospholidin-2-oxide 2c: Yield: 64.29%. M = 390g/mol [C24H27N2OP]. Mp. 178-179°C. Rf= 0.80 (CH2Cl2/MeOH). IR (KBr, cm-1): 1458.1 cm-1 (C=C Ar ), 1184.2 cm-1 (P=O), 1107.1 cm-1 (C-N-P). 1H NMR (CDCl3, 250MHz): 7.75 (m, 2H, H-Ar); 7.5 (m, 3H, H-Ar); 7.4-7.0 (m, 10H, H-Ar); 4.70 (m, 1H,*CH-NH); 4.40 (m, 1H,*CH-NH); 2.8 (t , J1= 9.34 Hz, J2= 9.48 Hz, 2H, CH2-cyc à 5); 2.7 (t , J1= 8.82 Hz, J2= 8.57 Hz, 2H, CH2-cyc à 5); 1.45 (d, J= 6 Hz, 3H, CH3); 1.30 (d, J=6.76 Hz, 3H, CH3). 31P NMR (CDCl3, 200.7MHz) δ = 34.64 ppm. 13C NMR (CDCl3, 250MHz): 138.3 (C-Ar); 134.2 (C-Ar); 134.1 (CH-Ar); 132.3 (CH-Ar); 128.8 (CH-Ar); 128.5 (CH-Ar); 127.9 (CH-Ar); 127 (CH-Ar); 45.6 (CH); 43.4 (CH2); 20.8 (CH3).1, 3-propyl, 2-phenyl diazaphospholidin-2-oxide 2d: Yield: 68.31%. M = 266 g /mol [C14H23N2OP]. Mp. 168-170°C. Rf= 0.82 (CH2Cl2/MeOH). IR (KBr, cm-1): 1451.4 cm-1 (C=C Ar ), 1192.9 cm-1 (P=O), 1029.3 cm-1 (C-N-P). 1H NMR (CDCl3, 250MHz): 7.80 (m, 2H, H-Ar); 7.65 (m, 3H, H-Ar); 3.0 (m,4H, N-CH2-CH2); 2.5 (t, J1= 9.37 Hz, J2= 9.43 Hz,4H, CH2 cyc à 5); 1.55 (m, 4H, -CH2-CH3);0.95 (t, J1= 9.35Hz, J2= 9.49 Hz, 6H, CH3). 31P NMR (CDCl3, 200.7MHz) δ = 33.7 ppm. 13C NMR (CDCl3, 250MHz): 134.6 (C-Ar); 131.5 (CH-Ar); 128.3 (CH-Ar); 128.3 (CH-Ar); 77 (CH2-NH); 42.57(CH2); 25.29 (CH2-); 11.28 (CH3).
4. Conclusions
- This work is based essentially on the synthesis of a new family of modified diazaphospholidines. We achieved the synthesis of phenyl phosphoramidates derived from primary amines (cyclohexylamine, benzylamine, propylamine, and ethylphenylamine) with phenyl phosphonic dichloride in one step. Our toxicological results showed a perturbation of respiratory metabolism, confirming the effect of these molecules on the respiratory chain. Thus we can conclude that the xenobiotics tested are cytotoxic. This toxicity is manifested by the loss of linearity of the trajectory and mobility followed by an inhibition of cell growth.
ACKNOWLEDGMENTS
- This work was generously supported by the (Direction Generale de la Recherche Scientifique et du Développement Technologique, DGRS-DT), Algerian Ministry of Scientific Research, (FNR) and CMEP 08 MDU 729.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML