-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2012; 2(1): 1-8
doi:10.5923/j.ajoc.20120201.01
Synthesis and Spectral Characterization of Novel 2, 3-Disubstituted Quinazolin-4(3H) one Derivatives
Mahmoud. R. Mahmoud, Wael. S. I. Abou-Elmagd, Salwa. S. Abdelwahab, El-Sayed. A. Soliman
Chemistry Department, Faculty of Science, Ain Shams University, Abassia, Cairo, post code 11566, Egypt
Correspondence to: Wael. S. I. Abou-Elmagd, Chemistry Department, Faculty of Science, Ain Shams University, Abassia, Cairo, post code 11566, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
New series of 2,3-disubstituted quinazolin-4(3H)- one were synthesized via the reaction of the readily obtainable 2-thioxo-3-phenyl-quinazolin-4(3H)one 1 with ethyl chloroacetate followed by hydrazinolysis to afford the hydrazide 3 which allowed to react with different electrophilic reagents such as carbon disulphide, phenyl isothiocyanate, β-diketones, anhydrides, acrylonitrile, ethyl cinnamate, dimethyl acetylene dicarboxylate, aldehydes, arylidene malononitrile and lauroyl chloride. Some of the newly synthesized compounds showed promising anti-inflammatory activity.
Keywords: Pyrzolyl-, 1,3,4-Oxadiazolyl Quinazolinone, Anti-Inflammatory, Michael Reaction
Cite this paper: Mahmoud. R. Mahmoud, Wael. S. I. Abou-Elmagd, Salwa. S. Abdelwahab, El-Sayed. A. Soliman, Synthesis and Spectral Characterization of Novel 2, 3-Disubstituted Quinazolin-4(3H) one Derivatives, American Journal of Organic Chemistry, Vol. 2 No. 1, 2012, pp. 1-8. doi: 10.5923/j.ajoc.20120201.01.
Article Outline
1. Introduction
- Several Quinazolinone derivatives were synthesized as potential antimicrobial[1,2], anticancer[3-7], anti-inflame- matory[8-11], and antimalarial[12] agents. Quinazolin-4(3H)one is a frequently encountered unit in natural products such as L-vasicinone[13], chrysogine[14,15] and drugs as Methaqualone[16], i.e. molecules based on quinazoline and quinazolinone exhibit a multitude of interesting pharmacological activities[17-19].
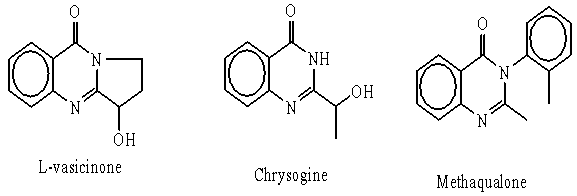 Aforementioned findings promoted the authors to synthesis a varieties of 2, 3-disubstituted quinazolinone derivatives via the utility of the key starting material 2-thioxo- 3-phenyl quinazolin 4(3H)one1[20,21].
Aforementioned findings promoted the authors to synthesis a varieties of 2, 3-disubstituted quinazolinone derivatives via the utility of the key starting material 2-thioxo- 3-phenyl quinazolin 4(3H)one1[20,21].2. Results and Discussion
- In this investigation, Ethyl-2-(4-oxo-3-phenyl-3, 4- dihydroquinazolin-2-yl thio)acetate 2 was obtained in fairly good yield upon treatment of quinazolin-2-thione derivative 1 with ethyl chloroacetate , in the presence of fused so The structure of compound 2 was confirmed from the study of its spectral data (c.f. Exp.). The highest recorded peak at m/z = 340 (34.21%) represent the molecular ion peak.In previous work[22], the reaction of ethyl S-(heteryl) thioglycollate with hydrazine in refluxing ethanol involves the elimination of this group and substituted by the hydrazine group.
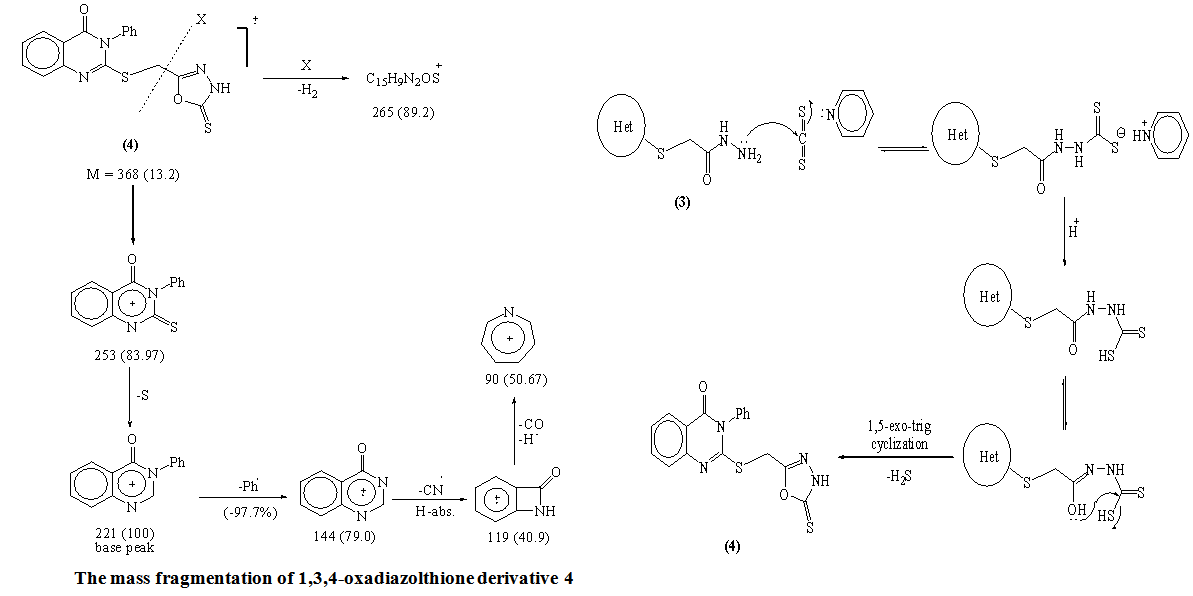
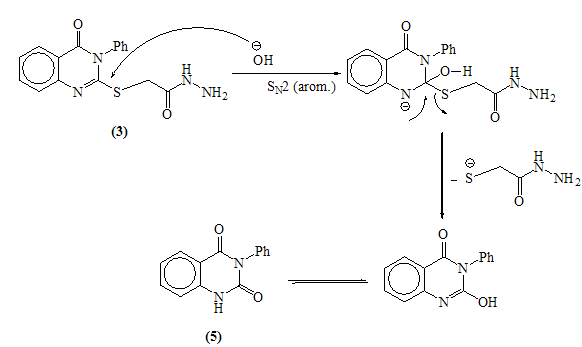
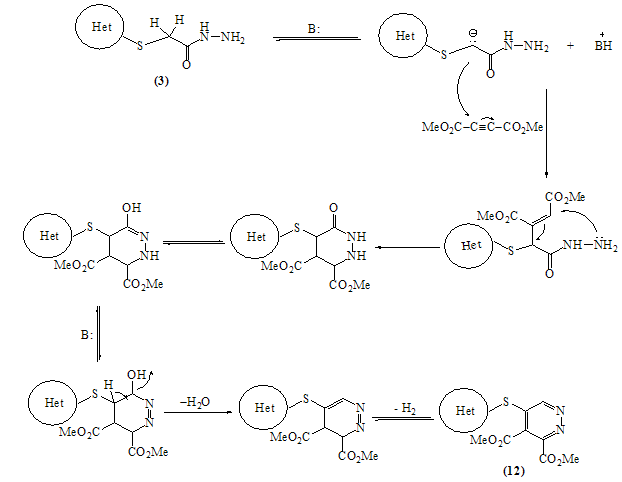
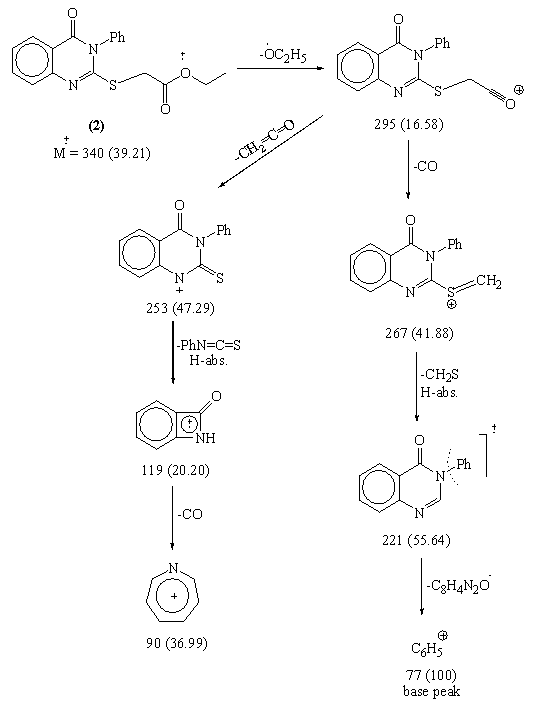 Mass fragmentation pattern of S-Alkylation product 2When compound 2 was allowed to react with hydrazine hydrate afforded the 2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio) acetohydrazide 3 which obtained via the nucleophilic nitrogen attack of the hydrazine moiety to the carbonyl group of the ester group through tetrahedral mechanism.The structure of 3 was established through spectroscopic (IR, 1HNMR and MS) beside the correct elemental analysis. (c.f. Exp)3-Phenyl-2-[(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl thio]quinazolin-4(3H)one 4 was obtained in fairly good yield upon refluxing the hydrazide 3 with carbon disulphide in pyridine on water bath for 8hrs.The structure 4 was deduced from the correct analytical and spectroscopic data (IR, 1HNMR). Full analysis for the mass spectrum of 4 shows the correct molecular ion peak at m/z = 368 (13.2%).The reaction is smoothly proceeded through tetrahedral pathway followed by 1,5-exo-trig cyclization to give 4. (Scheme 2)
Mass fragmentation pattern of S-Alkylation product 2When compound 2 was allowed to react with hydrazine hydrate afforded the 2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-ylthio) acetohydrazide 3 which obtained via the nucleophilic nitrogen attack of the hydrazine moiety to the carbonyl group of the ester group through tetrahedral mechanism.The structure of 3 was established through spectroscopic (IR, 1HNMR and MS) beside the correct elemental analysis. (c.f. Exp)3-Phenyl-2-[(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl thio]quinazolin-4(3H)one 4 was obtained in fairly good yield upon refluxing the hydrazide 3 with carbon disulphide in pyridine on water bath for 8hrs.The structure 4 was deduced from the correct analytical and spectroscopic data (IR, 1HNMR). Full analysis for the mass spectrum of 4 shows the correct molecular ion peak at m/z = 368 (13.2%).The reaction is smoothly proceeded through tetrahedral pathway followed by 1,5-exo-trig cyclization to give 4. (Scheme 2) 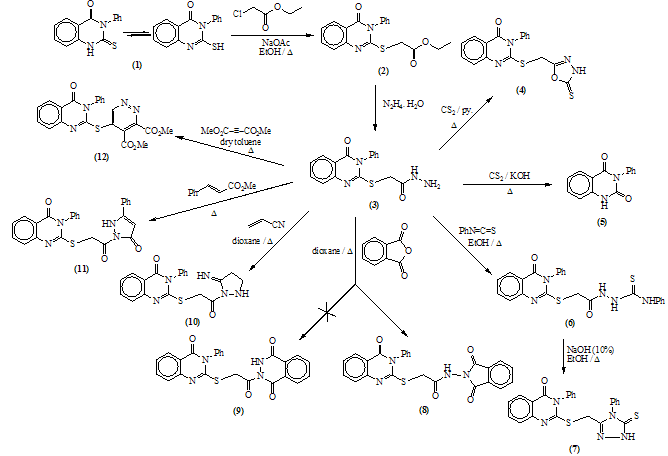 | Scheme 1. |
 | Scheme 2. |
 | Scheme 3. |
 | Scheme 4. |
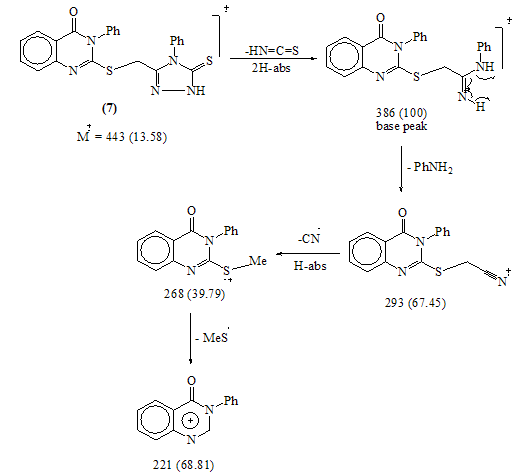 EI-MS of compound 7The structure of 8 was confirmed from the correct analytical and spectroscopic data. No evidence for the formation of the phthalazine derivative 9 was detected, since, the IR spectrum of the product shows the carbonyl vibrational coupling bands at 1793 and 1738cm-1 together with υC=O (quinazolinone) at 1684 cm-1 which agree well with the structure 8.(c.f.Exp.)The mass spectrum of 8 show the correct molecular ion peak at m/z = 456 (4.04 %) which upon loss of N- aminophthalimide radical afforded the base peak at m/z = 295 (100%). Refluxing compound 3 with acrylonitrile in dioxane afforded 2-[2-(5-iminopyrazolidin-1-yl)-2-oxoethyl thio]-3- phenylquinazolin-4(3H) one 10. The IR spectrum of 10 revealed the absence of the stretching absorption band for the nitrile group which indicates that the nitrile group involved in the cyclization process. The EI-MS is completely in accord with the assigned structure. (c.f. Exp.) Fusion of 3 with methylcinnamate on oil bath at 180 ᵒC yielded a crude solid product which triturated with ethanol to give 2-[2- oxo-2-(5-oxo-3-phenyl-Δ3 pyrazolin-1-yl)ethyl thio]-3-phe- nylquinazolin-4(3H)one 11.The structure 11 was established by the spectroscopic and analytical data. 1H-NMR spectrum of compound 11 (CDCl3) lacked the signals attributable for ABX system -CH-CH2- which indicate the dehydrogenation during the reaction conditions. Ample evidence for the structure 11 is forthcoming from the analysis of the mass spectrum in which the highest recorded peak at m/z = 455 (64.9%) attributable for the M+1 radical cation. (c.f. Exp.)The reaction is smoothly proceeded via Michael addition followed by 1,5-exo-trig cyclization and dehydrogenation. (Scheme 5)
EI-MS of compound 7The structure of 8 was confirmed from the correct analytical and spectroscopic data. No evidence for the formation of the phthalazine derivative 9 was detected, since, the IR spectrum of the product shows the carbonyl vibrational coupling bands at 1793 and 1738cm-1 together with υC=O (quinazolinone) at 1684 cm-1 which agree well with the structure 8.(c.f.Exp.)The mass spectrum of 8 show the correct molecular ion peak at m/z = 456 (4.04 %) which upon loss of N- aminophthalimide radical afforded the base peak at m/z = 295 (100%). Refluxing compound 3 with acrylonitrile in dioxane afforded 2-[2-(5-iminopyrazolidin-1-yl)-2-oxoethyl thio]-3- phenylquinazolin-4(3H) one 10. The IR spectrum of 10 revealed the absence of the stretching absorption band for the nitrile group which indicates that the nitrile group involved in the cyclization process. The EI-MS is completely in accord with the assigned structure. (c.f. Exp.) Fusion of 3 with methylcinnamate on oil bath at 180 ᵒC yielded a crude solid product which triturated with ethanol to give 2-[2- oxo-2-(5-oxo-3-phenyl-Δ3 pyrazolin-1-yl)ethyl thio]-3-phe- nylquinazolin-4(3H)one 11.The structure 11 was established by the spectroscopic and analytical data. 1H-NMR spectrum of compound 11 (CDCl3) lacked the signals attributable for ABX system -CH-CH2- which indicate the dehydrogenation during the reaction conditions. Ample evidence for the structure 11 is forthcoming from the analysis of the mass spectrum in which the highest recorded peak at m/z = 455 (64.9%) attributable for the M+1 radical cation. (c.f. Exp.)The reaction is smoothly proceeded via Michael addition followed by 1,5-exo-trig cyclization and dehydrogenation. (Scheme 5)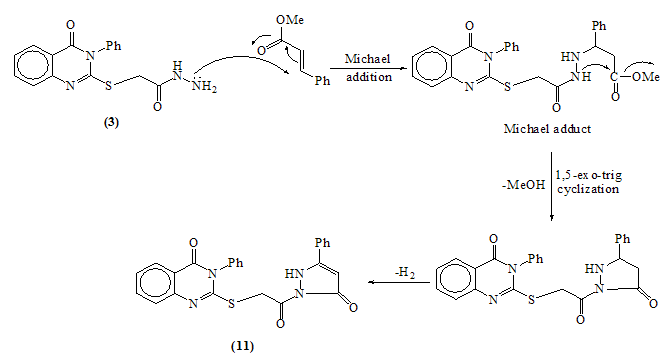 | Scheme 5. |
 | Scheme 6. |
 | Scheme 7. |
3. Experimental
- Melting points are measured on an electrothermal melting point apparatus. Elemental analyses were carried out at the microanalytical unit, Cairo Univeristy. The IR spectra were measured on a Unicam SP-1200 spectrometer using KBr Wafer technique. The 1H-NMR spectra were measured in DMSO-d6 on a Varian plus instrument (300MHz). Mass spectra were recorded on a shimadzu GC-MS QP- 1000EX instrument operating at 70 ev.
3.1. Synthesis of S-(Heteryl)Thioglycollate 2
- To a solution of 1 (2.54g, 0.01mol) and ethylcloroacetate (1.23g, 0.01mol) in (50ml) ethanol, fused sodium acetate (0.8g, 0.01mol) was added. The whole mixture was refluxed for 6hrs, the whole mixture was concentrated and left to cool. The precipitated solid was filttered off, washed with water, dried and recrystallized from ethanol to give ethyl-2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl thio)acetate 2. Colorless crystals (70% yield); m.p: 105-107°C. IR: υmax 1739 (C=Oester), 1690 (C=Oquinazolin) cm-1. 1H-NMR (CDCl3): δ (ppm) 1.31 (t, 3H, CH3-CH2, J=7.2Hz), 3.91 (s, 2H, CH2-CO), 4.24 (q, 2H, CH3-CH2, J=7.2Hz), 7.27-8.26 (m, 9H, ArH). MS: m/z (%) 340 (M+, 39), 295 (17), 294 (15), 267 (42), 266 (21), 253 (48), 165 (25), 221 (56), 119 (21), 90 (37), 77 (100), 63 (16). Anal. Calcd. for C18H16N2O3S (340): C, 63.52; H, 4.70; N, 8.23; S, 9.41. Found C, 63.76; H, 4.95; N, 8.51; S, 9.79.
3.2. Hydrazinolysis of 2; Formation of Hydrazide 3
- A mixture of 2 (2.5g, 0.008mol) and hydrazine hydrate (0.4g, 0.008mol) was stirred in (50ml) ethanol for 10hrs. The precipitated solid was filttered off and recrystallized from toluene to give 2-(4-oxo-3-phenyl-3, 4-dihydroquinazolin-2-yl thio)acetohydrazide 3. Colorless crystals (65% yield); m.p: 180-183°C. IR: υmax 3303, 3250, 3220 (NH, NH2), 1693 (C=Oquinazolin), 1663 (C=Ohydrazide) cm-1. 1H-NMR (DMSO-d6): δ (ppm) 3.83 (s, 2H, CH2-CO), 4.25 (br.s, 2H, NH2, exchangeable), 7.26-8.10 (m, 9H, ArH), 9.29 (br.s, 1H, NH-CO, exchangeable). MS: m/z (%) 326 (M.+, 7), 296 (21), 295 (100), 254 (22), 253 (81), 221 (23), 132 (43), 90 (27), 77 (70). Anal. Calcd. for C16H14N4O2S (326): C, 58.88; H, 4.32; N, 17.17; S, 9.82. Found C, 58.49; H, 4.10; N, 16.99; S, 9.60.
3.3. Reaction of the Hydrazide 3 with Carbon Disulphide in Pyridine; Formation of Oxadiazolthione Derivative 4
- A solution of hydrazide 3 (0.65g, 0.002mol) in pyridine (50ml) and carbon disulphide (1.5ml, 0.02mol) were refluxed on water bath for 7hrs. The cooled concentrated solution was poured onto ice-water (50ml) and acidified with acetic acid (2ml). The mixture was extracted twice with chloroform (75ml). The organic layer was dried, concentrated, left for slow evaporation. The crude precipitated was crystallized from light petroleum to give 3-phenyl-2- ((5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)methylthio)quinazolin-4(3H)-one 4. Yellow crystals (67% yield); m.p: 210-213°C. IR: υmax 3444, 3130 (NH), 1665 (C=Oquinazolin), 1262 (C=S) cm-1. 1H-NMR (DMSO-d6): δ (ppm) 4.53 (s, 2H, CH2-S-), 7.26-8.11 (m, 10H, 9ArH + NH exchangeable). MS: m/z (%)368 (M+, 14), 266 (26), 264 (89), 253 (84), 230 (86), 221 (100), 167 (45), 144 (39), 143 (79), 119 (41), 91 (35), 77 (98), 63 (16). Anal. Calcd. for C17H12N4O2S2 (368): C, 55.42; H, 3.28; N, 15.21; S, 17.41. Found C, 55.89; H, 3.41; N, 15.09; S, 17.67.
3.4. Reaction of Hydrazide 3 with Carbon Disulphide in Ethanolic Potassium Hydroxide; Formation of Quinazolindione Derivative 5
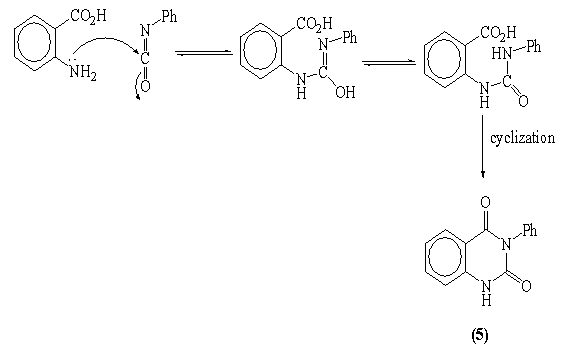
- A solution of hydrazide 3 (0.65g, 0.002mol) in ethanol (50ml), potassium hydroxide (0.1g, 0.002mol) and excess of carbon disulphide (1.5ml, 0.02mol) were refluxed on water bath for 7hrs. The cooled concentrated solution was poured onto ice-water (50ml) and acidified with acetic acid (2ml). The crude product was filtered off, dried and recrystallized from ethanol to give 3-phenyl quinazolin-2,4(1H, 3H)-dione 5. Yellow crystals (60% yield); m.p: 260-261°C[23]. IR: υmax 3368 (NH), 1731,1652 (C=O) cm-1. MS: m/z (%)238 (M.+,100), 146 (42), 119(77), 92 (12). Anal. Calcd. for C14H10N2O2 (238): C, 70.58; H, 4.23; N, 11.76. Found C, 70.71; H, 4.40; N, 11.99.
3.5. Formation of Phenyl Isothio Semicarbazide Derivative 6
- A mixture of hydrazide 3 (0.65g, 0.002mol) and phenyl isothiocyanate (0.2ml, 0.002mol) was refluxed in ethanol (30 ml) for 1hr. The reaction mixture was left to cool, the crude solid was filtered off, washed twice with cold ethanol, dried and recrystallized from ethanol to give 1-(2- (4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl thio)acetyl)-4- phenyl thiosemicarbazide 6. Yellow crystals (70% yield); m.p: 210-212°C. IR: υmax 3316, 3274, 3179 (NH), 1688 (C=Oquinazolin), 1657 (C=Oamide), 1617 (C=N), 1260 (C=S) cm-1. MS: m/z (%) 461 (M.+, 22), 420 (24), 367 (25), 312 (31), 295 (49), 294 (100), 293 (30), 260 (33), 253 (74), 239 (26), 180 (32), 150 (47), 134 (55), 94 (34). Anal. Calcd. for C23H19N5O2S2 (461): C, 59.85; H, 4.15; N, 15.17; S, 13.89. Found C, 60.09; H, 4.31; N, 15.38; S, 13.57.
3.6. Cyclization of Compound 6; Formation of Triazolthione Derivative 7
- A solution of 6 (0.7g, 0.0015mol) in ethanolic sodium hydroxide (0.25g in 20ml ethanol) was heated under reflux for 3hrs and then left to cool. The solid obtained was filtered off, washed with water and recrystallized from ethanol to give 3-phenyl-2-((4-phenyl-5-thioxo-4, 5-dihydro-1H-1, 2, 4-triazol-3-yl)methylthio)quinazolin-4(3H)-one 7. Yellow crystals (79% yield); m.p >300°C. IR: υmax 3392 (NH), 1688 (C=Oquinazolin), 1247 (C=S) cm-1. 1H-NMR (DMSO-d6): δ (ppm) 3.90 (s, 2H, CH2-S-), 6.62-7.98 (m, 15H, 14ArH + NH exchangeable). MS: m/z (%)443 (M+, 14), 388 (21), 386 (100), 353 (24), 284 (41), 268 (40), 221 (69), 186 (46), 159 (43), 134 (72). Anal. Calcd. for C23H17N5OS2 (443): C, 62.28; H, 3.86; N, 15.79; S, 14.46. Found C, 62.17; H, 3.93; N, 15.90; S, 14.80.
3.7. Reaction of hydrazide 3 with Phthalic Anhydride; Formation of N-Substituted Isoindolindione Derivative 8
- A mixture of hydrazide 3 (0.65g, 0.002mol) and phthalic anhydride (0.2g, 0.002mol) wasrefluxed in dry dioxane (20ml) for 5hrs. The reaction mixture was left overnight for slow evaporation. The crude product was recrystallized twice from light petroleum /toluene mixture to give N-(1,3-dioxoisoindolin-2-yl)-2-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl thio)acetamide 8. Yellowish white crystals (50% yield); m.p: 250-252°C. IR: υmax 3185 (NH), 1793, 1738, 1684 (C=O) cm-1. 1H-NMR (DMSO-d6): δ (ppm) 3.99 (s, 2H, CH2-S-), 7.27-7.89 (m, 13H, ArH), 9.82 (s, 1H, NH, exchangeable). MS: m/z (%) 456 (M+, 4), 296 (27), 295 (100), 253 (17), 221 (21), 132 (39), 104 (48), 89 (20). Anal. Calcd. for C24H16N4O4S (456): C, 63.15; H, 3.53; N, 12.27; S, 7.02. Found C, 63.27; H, 3.68; N, 12.08; S, 6.90.
3.8. Synthesis of Iminopyrazolidin Derivative 10
- A mixture of hydrazide 3 (0.65g, 0.002mol), acrylonitrile ( 0.114g, 0.002mol) 1n pyridine (20ml) was refluxed for 3hrs. The reaction mixture was left overnight for slow evaporation. The formed solid residue was recrystallized from light petroleum-toluene mixture to give 2-(2-(5-iminopyrazolidin-1-yl)-2-oxoethyl thio)-3- phenyl quinazolin-4(3H)-one 10. Yellow crystals (70% yield); m.p: 224-226°C. IR: υmax 3190 (NH), 1687 (C=O) cm-1. MS: m/z (%)379 (M+, 14), 295 (31), 254 (49), 253 (90), 235 (30), 225 (16), 221(49), 181 (21), 167 (90), 166 (50), 162 (36), 145 (31), 144 (100), 134 (20), 132 (28), 119 (45), 92 (40), 90 (59), 77 (75). Anal. Calcd. for C19H17N5O2S (379): C, 60.14; H, 4.52; N, 18.46; S, 8.45. Found C, 60.39; H, 4.87; N, 18.79; S, 8.20.
3.9. Reaction of 3 with Methyl Cinnamate; Formation of Pyrazolidinone Derivative 11
- A mixture of hydrazide 3 (0.65g, 0.002mol) and methyl cinnamate (0.23g, 0.002mol) was fused in sand bath for 4hrs at 180-190°C. The residue was treated with hot light petroleum/toluene mixture (50ml). The formed crystals were collected, dried and crystallized from toluene to give 2-(2-oxo-2-(5-oxo-3-phenyl-2H-pyrazol-1(5H)-yl) ethyl thio)-3-phenyl quinazolin-4(3H)-one 11. Yellow crystals (56% yield); m.p: 258-260°C. IR: υmax 2220 (NH), 1690, 1641 (C=O) cm-1. 1H-NMR (DMSO-d6): δ (ppm) 3.78 (s, 2H, CH2-S-), 7.26-8.23 (m, 15H, ArH), 10.71 (br.s, 2H, NH, exchangeable). MS: m/z (%)454 (M+, 65), 253 (93), 231 (76), 221 (92), 145 (67), 143 (79), 134(68), 109 (67), 105 (67), 85 (67), 77 (83), 75 (91), 62 (75), 61 (100). Anal. Calcd. for C25H18N4O3S (454): C, 66.07; H, 3.99; N, 12.33; S, 7.05. Found C, 66.29; H, 4.15; N, 12.70; S, 7.19.
3.10. Synthesis of Pyridazine Dicarboxylate Derivative 12
- A solution of hydrazide 3 (0.65g, 0.002mol)and (0.22g, 0.002mol) dimethyl acetylene dicarboxylate in dioxane (20ml) was refluxed for 6hrs. The reaction mixture was left overnight for slow evaporation. The crude precipitate was crystallized from benzene to give dimethyl-5-(4-oxo-3- phenyl-3,4-dihydroquinazolin-2-yl thio)-pyridazin-3,4- dicarboxylate 12. Yellowish white crystals (60% yield); m.p: 127-129°C. IR: υmax 3437, 3261 (NH), 1734, 1689 (C=O) cm-1. 1H-NMR (DMSO-d6): δ (ppm) 3.64 (s, 3H, -COOCH3) , 3.78 (s, 3H, -COOCH3), 7.08-7.81 (m, 9H, ArH), 8.07 (s, 1H, C6-Hpyridazin). MS: m/z (%)448 (M+, 20), 435 (17), 423 (15), 409 (80), 394 (17), 379 (21), 362(19), 295 (25), 253 (16), 295 (65), 267 (14), 254 (28), 253 (59), 235 (16), 221 (41), 191 (34), 181 (43), 119 (23), 111 (37), 110 (44), 91 (50), 77 (100). Anal. Calcd. for C22H16N4O5S (448): C, 58.92; H, 3.60; N, 12.49; S, 7.15. Found C, 59.17; H, 3.81; N, 12.69; S, 7.40.
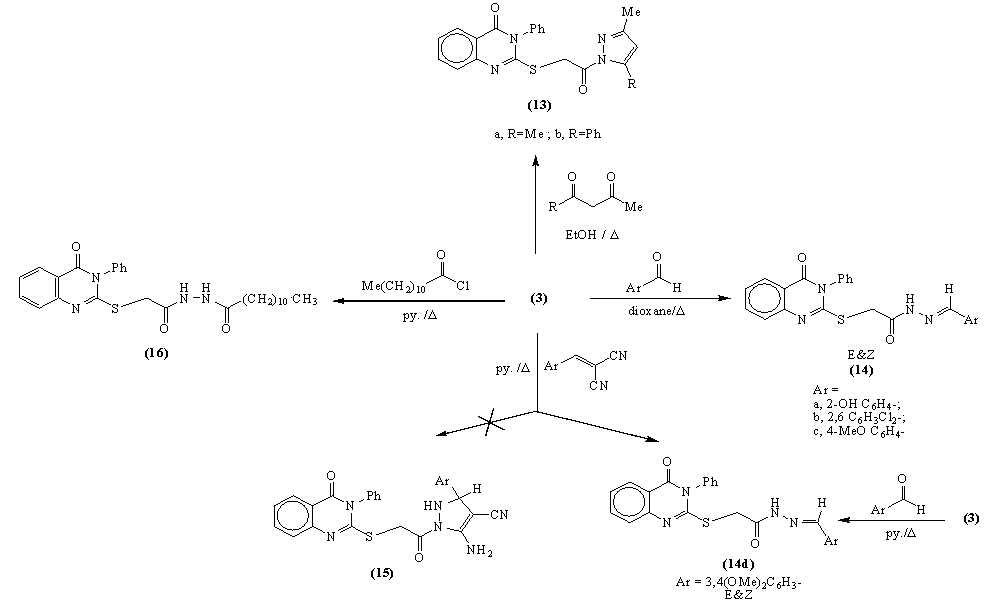
3.11. General Procedure for the Reaction of Hydrazide 3 with Acetyl Acetone or Benzoyl Acetone; Formation of Pyrazol Derivative 13
- A mixture of hydrazide 3 (0.65g, 0.002mol), acetyl acetone (0.2ml, 0.002mol) or benzoyl acetone (0.3g, 0.002mol) was refluxed in ethanol (50ml) for 7hrs. The reaction mixture was left overnight for slow evaporation. The solid product was collected, filtered off, dried and recrystallized from light petroleum and chloroform to give 13a and 13b respectively.
3.11.1. 2-(2-(3,5-dimethyl-1H-pyrazol-1-yl)-2-oxo ethylth- io)-3-phenyl quinazolin-4(3H)-one 13a
- Pale Yellow crystals (78% yield); m.p: 76-78°C. IR: υmax 1688 (C=Oquinazolin), 1644 (C=Oamide) cm-1. 1H-NMR (CDCl3): δ (ppm) 1.82 (s, 3H, C5-Me), 2.08 (s, 3H, C3-Me), 4.13(s, 2H, CH2-S) 7.2-7.6 (m, 9H, ArH), 8.2 (s, 1H, C4-Hpyrazole). MS: m/z (%)390 (M+, 8), 296 (27), 295 (100), 294 (33), 255 (13), 253 (24), 221(24), 132 (12). Anal. Calcd. for C21H18N4O2S (390): C, 64.60; H, 4.65; N, 14.35; S, 8.25. Found C, 64.97; H, 4.37; N, 14.72; S, 8.53.
3.11.2. 2-(2-(3-methyl-5-phenyl-1H-pyrazol-1-yl)-2-oxo et- hylthio)-3-phenyl quinazolin-4(3H)-one 13b
- Bright Yellow crystals (55% yield); m.p: 166-168°C. IR: υmax 1689 (C=Oquinazolin), 1652 (C=Oamide) cm-1. MS: m/z (%)452 (M+, 10), 439 (20), 411 (19), 403 (23), 340 (46), 328 (40), 307(52), 294 (42), 253 (100), 235 (65), 221 (66), 162 (64), 149 (80), 147(66), 132 (47), 104 (49), 97 (53). Anal. Calcd. for C26H20N4O2S (452): C, 69.01; H, 4.45; N, 12.38; S, 7.09. Found C, 69.30; H, 4.72; N, 12.19; S, 6.95.
3.12. General Procedure for the Reaction of Hydrazide 3 with Aromatic Aldehydes
- A solution of hydrazide 3 (0.65g, 0.002mol) in dioxane (30 ml) and salicylaldehyde (0.2g, 0.002mol) or 2,6-dichlorobenzaldehyde (0.3g, 0.002mol) or p- anisaldehyde (0.12g, 0.002mol) was refluxed in presence of (0.2ml) triethylamine for 24h. The precipitate formed was dried and recrystallized from light petroleum-toluene mixture to give N′-arylidene-2-(4-oxo-3-phenyl-3, 4-dihydroquinazolin-2-yl thio)acetohydrazide 14a, 14b and 14c respectively.
3.12.1. N′-(2-hydroxybenzylidene)-2-(4-oxo-3-phenyl-3, 4-dihydroquinazolin-2-ylthio) acetohydrazide 14a
- Yellow crystals (85% yield); m.p: 215-217°C. IR: υmax 3444 (OH), 3177 (NH), 1678 (C=Oquinazolin), 1655 (C=Oamide) cm-1. 1H-NMR (DMSO-d6): δ (ppm) (E-form, 55%) 4 (s, 2H, -CH2-S), 6.89-8.09 (m, 13H, ArH), 8.47 (s, 1H, =CH), 10.99 (s, 1H, -OH, exchangeable), 11.95 (s, 1H, NH, exchangeable); (Z-form, 45%) 8.34 (s, 1H, =CH), 10.08 (s, 1H, -OH, exchangeable), 11.60 (s, 1H, NH, exchangeable). MS: m/z (%)430 (M+, 8), 297 (10), 296 (21), 295 (100), 253 (30), 132 (40), 77(48). Anal. Calcd. for C23H18N4O3S (430): C, 64.17; H, 4.21; N, 13.01; S, 7.45. Found C, 64.40; H, 4.45; N, 12.89; S, 7.67.
3.12.2. N′-(2,6-dichlorobenzylidene)-2-(4-oxo-3-phenyl-3, 4-dihydroquinazolin-2-ylthio) acetohydrazide 14b
- Yellow crystals (80% yield); m.p: 208-210°C. IR: υmax 3189 (NH), 1684 (C=Oquinazolin), 1657 (C=Oamide) cm-1. 1H-NMR (DMSO-d6): δ (ppm) (E-form, 82%) 4.43 (s, 2H, -CH2-S), 7.43-8.08 (m, 12H, ArH), 8.31 (s, 1H, =CH), 11.87 (s, 1H, NH, exchangeable); (Z-form, 18%) 8.45 (s, 1H, =CH), 12.02 (s, 1H, NH, exchangeable). MS: m/z (%) 482 (M+, 0.86), 451 (15), 409 (10), 311 (17), 297 (43), 295 (100), 268(18), 264 (15), 221 (17), 132 (43), 91 (18), 90 (22), 77(47). Anal. Calcd. for C23H16Cl2N4O3S (482): C, 57.15; H, 3.34; N, 11.59; S, 6.63. Found C, 57.40; H, 3.59; N, 11.77; S, 6.39.
3.12.3. (E)-N′-(4-methoxybenzylidene)-2-(4-oxo-3- phenyl-3,4-dihydroquinazolin-2-ylthio) acetohydrazide 14c
- Yellow crystals (82% yield); m.p: 217-219°C. IR: υmax 3172 (NH), 1680 (C=Oquinazolin), 1645 (C=Oamide) cm-1. MS: m/z (%)444 (M+, 20), 296 (20), 295 (100), 253 (25), 221 (16), 144 (29), 132 (26), 120 (23), 104 (18), 91 (30), 77(38). Anal. Calcd. for C24H20N4O3S (444): C, 64.85; H, 4.54; N, 12.60; S, 7.21. Found C, 65.19; H, 4.80; N, 12.93; S, 7.09.
3.13. The Reaction of Hydrazide 3 with 2-(3, 4- dimethoxybenzylidene)Malononitrile. Formation of 14d
- A solution of hydrazide 3 (0.65g, 0.002mol) and 2-(3,4-dimethoxy benzylidene)malononitrile (0.2g, 0.002 mol) in pyridine (10ml) was refluxed for 8hrs. The reaction mixture was acidified with ice cold hydrochloric acid. The solid product obtained was filtered off and recrystallized from ethanol to give 14d as a mixture of E&Z isomers. The same product 14d was obtained when the hydrazide 3 refluxed with 3,4-dimethoxy benzaldehyde in pyridine.
3.13.1. N′-(3,4-dimethoxybenzylidene)-2-(4-oxo-3- phenyl-3,4-dihydroquinazolin-2-ylthio) acetohydrazide 14d
- Yellow crystals (50% yield); m.p: 220-222°C. IR: υmax 3180 (NH), 1681 (C=Oquinazolin), 1645 (C=Oamide) cm-1. 1H-NMR (CDCl3): δ (ppm) (E-form, 75%) 3.88 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 4.5 (s, 2H, -CH2-S), 6.81-8.34 (m, 12H, ArH), 8.93 (s, 1H, =CH), 10.79 (s, 1H, NH, exchangeable); (Z-form, 25%) 3.79 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 8.89 (s, 1H, =CH), 10.75 (s, 1H, NH, exchangeable). Anal. Calcd. for C25H22N4O4S (474): C, 63.28; H, 4.67; N, 11.81; S, 6.76. Found C, 63.51; H, 4.92; N, 11.59; S, 6.55.
3.14. Acylation of 3 Using Lauroyl Chloride
- A mixture of hydrazide 3 (0.33 g, 0.001 mol) in dry pyridine (20 ml) and lauroyl chloride (0.22 g, 0.001 mol) were refluxed for 4h. The reaction mixture poured onto ice cold acetic acid. The solid product was filterd off and recrystallized from petroleum ether/toluene mixture to give N-acylated product 16.
3.14.1. N′-(2-(4-oxo-3-phenyl-3, 4-dihydroquinazolin-2-yl thio)acetyl)dodecane hydrazide 16
- Yellowish white crystals (65% yield); m.p: 120-121°C. IR: υmax 3210, 3195 (NH), 1701 (C=Oquinazolin), 1650 (C=Oamide) cm-1. 1H-NMR (DMSO-d6): δ (ppm) 0.87 (t, 3H, CH3, J = 6.6Hz), 1.24-1.48 (m, 18H, 9 CH2), 2.05-2.17 (m, 2H, CH2CO), 3.94 (s, 2H, CH2-S), 7.44-8.01 (m, 9H, ArH), 9.80 (s, 1H, NH, exchangeable), 10.1 (s, 1H, NH exchangeable). Anal. Calcd. for C28H36N4O3S (508): C, 66.11; H, 7.13; N, 11.01; S, 6.30. Found C, 66.40; H, 7.39; N, 11.21; S, 6.17.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML