-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2011; 1(1): 10-13
doi: 10.5923/j.ajoc.20110101.03
Green, Two Components Highly Efficient Reaction of Ninhydrin with Aromatic Amines, and Malononitrile Using Ball-Milling Technique
H. A. Etman , H. M. Metwally , M. M. Elkasaby , A. M. Khalil , M. A. Metwally
Chemistry Department, Faculty of Science, Mansoura University, Mansoura, Egypt
Correspondence to: M. A. Metwally , Chemistry Department, Faculty of Science, Mansoura University, Mansoura, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Ninhydrin (1) underwent several reactions with benzidine (2), o-phenylene diamine (4), p-toluidine (6) and malononitrile (9) for the purpose of producing novel indanedione derivatives of expected biological activity.
Keywords: Green Chemistry, Ninhydrin, Aromatic Amines, Condensation Reactions
Cite this paper: H. A. Etman , H. M. Metwally , M. M. Elkasaby , A. M. Khalil , M. A. Metwally , "Green, Two Components Highly Efficient Reaction of Ninhydrin with Aromatic Amines, and Malononitrile Using Ball-Milling Technique", American Journal of Organic Chemistry, Vol. 1 No. 1, 2011, pp. 10-13. doi: 10.5923/j.ajoc.20110101.03.
1. Introduction
- The development of simple synthetic routes for widely used organic compounds from readily available reagents is one of the major tasks in organic synthesis. We report herein an efficient, solid-state synthesis of highly functionalized indan-1,3-diones via the reaction of ninhydrin with a variety of reagents as an extension to our previous work on the benign synthesis of these compounds [1a,b].
2. Results and Discussions
- The easy access of a hydroxyl group of ninhydrin (1) to its substitution and the presence of carbonyl groups makes this highly reactive compound an interesting start point for cascade reactions with amino compounds[2]. Benzidine 2 condensed quantitatively with ninhydrin 1 at room temperature (molar ratio 1:1) in the solid state (ball milling) to give bis-azomethine 3 in 100% yield. The potential of the ball milling can be achieved by comparing the yield % of the same product 3 obtained when the reaction was carried out in solution (EtOH – AcOH , ratio 7:3) or by grinding. The yield % in both cases reached to 60%The ball milling reaction of ninhydrin 1 with benzeidine 2 at room temperature afforded only the corresponding bis-azomethine 3 in a quantitative yield. The water of reaction was easily removed by heating to 80°C in a vacuum. The chemical structure of 3 was established on the basis of spectral data. The IR spectrum confirmed the presence of -C=N and C=O stretching absorption bands at 1608, and 1718 cm-1. The 1H-NMR spectrum revealed the ArH at 7.3-8 ppm. The mass spectrum showed a molecular ion peak (M+)at m/z = 469.04 (M++1), corresponding to the molecular weight of the molecular formula C30H16N2O4= 468.46.
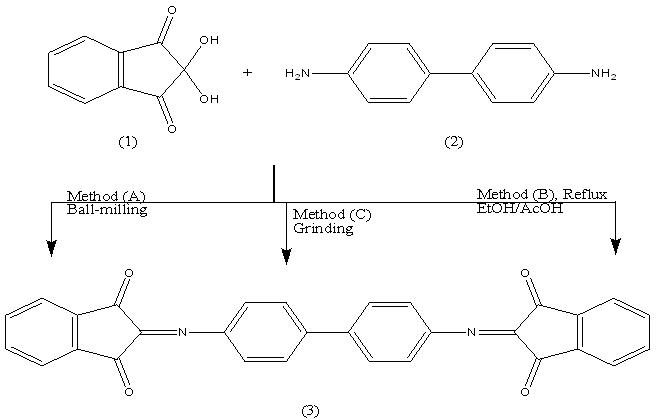 Reaction of ninhydrin 1 with an equimolar amount of benzidine 2 in EtOH/AcOH, (ratio 7:3) afforded the corresponding bis-azomethine 3 in 60% yield. The spectral data of azomethine 3 were in consistent with the structure. By analogy, grinding 1 and 2 in a mortar at room temperature for two hours resulted in the formation of 3 as inferred from its correct analytical and spectral data. In all three methods of synthesis, the m.p is the same.The technique of waste-free solid-state reaction could be applied to prepare the intramolecular bis-azomethine 5. It has been reported that stochiometric runs by the ball milling of ninhydrin 1 with o-phenylene diamine 4 afforded the corresponding pyrazine derivative 5 with 100% yield without the aid of any catalysts or solvents.The 4-cascades of 1 with o-phenylenediamine 4, reported by Kauppp[2] are fully regiospecific and also quantitative upon milling at -5°C and drying at 80°C in vacuum. Our method is an easy access to 5 with well-defined substitution at room temprature. A reasonable sequence of events is addition to C=O, substitution of OH and two eliminations of water
Reaction of ninhydrin 1 with an equimolar amount of benzidine 2 in EtOH/AcOH, (ratio 7:3) afforded the corresponding bis-azomethine 3 in 60% yield. The spectral data of azomethine 3 were in consistent with the structure. By analogy, grinding 1 and 2 in a mortar at room temperature for two hours resulted in the formation of 3 as inferred from its correct analytical and spectral data. In all three methods of synthesis, the m.p is the same.The technique of waste-free solid-state reaction could be applied to prepare the intramolecular bis-azomethine 5. It has been reported that stochiometric runs by the ball milling of ninhydrin 1 with o-phenylene diamine 4 afforded the corresponding pyrazine derivative 5 with 100% yield without the aid of any catalysts or solvents.The 4-cascades of 1 with o-phenylenediamine 4, reported by Kauppp[2] are fully regiospecific and also quantitative upon milling at -5°C and drying at 80°C in vacuum. Our method is an easy access to 5 with well-defined substitution at room temprature. A reasonable sequence of events is addition to C=O, substitution of OH and two eliminations of water 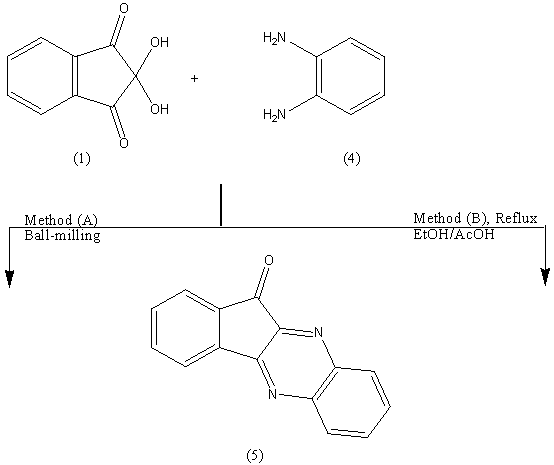 Further evidence for the formation of 5 was gained from its synthesis in (EtOH/AcOH, ratio 7:3) in 60% yield, mp and mixed mp. The formation of 5 finds support from the correct analytical and spectral data.The IR spectrum confirmed the presence of -C=N and C=O absorption bands at 1604, 1727 cm-1. The 1H-NMR spectrum revealed the ArH at δ 7.2-8.1 ppm. The mass spectrum showed a molecular ion peak (M+) at m/z = 232.33 corresponding to the molecular weight of the molecular formula C15H8N2O= 232.24.
Further evidence for the formation of 5 was gained from its synthesis in (EtOH/AcOH, ratio 7:3) in 60% yield, mp and mixed mp. The formation of 5 finds support from the correct analytical and spectral data.The IR spectrum confirmed the presence of -C=N and C=O absorption bands at 1604, 1727 cm-1. The 1H-NMR spectrum revealed the ArH at δ 7.2-8.1 ppm. The mass spectrum showed a molecular ion peak (M+) at m/z = 232.33 corresponding to the molecular weight of the molecular formula C15H8N2O= 232.24.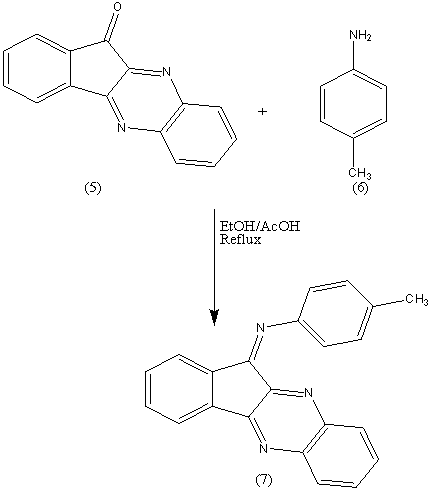 As a further extension for the above successful reactions, it seemed of interest to react the pyrazine 5 with p-tolidine 6 by refluxing in EtOH/AcOH (ratio 7:3) to give compound 7. The formation of 7 finds support from the correct analytical and spectral data. The IR spectrum confirmed the presence of -C=N absorption band at 1614 cm-1. The mass spectrum showed a molecular ion peak (M+) at m/z = 321.43 (100%) corresponding to the molecular weight of the molecular formula C22H15N3= 321.37.p-Toulidine 6 condensed quantitatively with ninhydrine 1 at room temperature (molar ratio 1:1) in the solid state (ball milling) to give the azomethine 8 in 100% yield. The potential of the ball milling can be achieved by comparing the yield % of the same product 3 obtained when the reaction was carried out in solution (EtOH – AcOH , ratio 7:3) or by grinding. The yield % in both cases reached to 60%.
As a further extension for the above successful reactions, it seemed of interest to react the pyrazine 5 with p-tolidine 6 by refluxing in EtOH/AcOH (ratio 7:3) to give compound 7. The formation of 7 finds support from the correct analytical and spectral data. The IR spectrum confirmed the presence of -C=N absorption band at 1614 cm-1. The mass spectrum showed a molecular ion peak (M+) at m/z = 321.43 (100%) corresponding to the molecular weight of the molecular formula C22H15N3= 321.37.p-Toulidine 6 condensed quantitatively with ninhydrine 1 at room temperature (molar ratio 1:1) in the solid state (ball milling) to give the azomethine 8 in 100% yield. The potential of the ball milling can be achieved by comparing the yield % of the same product 3 obtained when the reaction was carried out in solution (EtOH – AcOH , ratio 7:3) or by grinding. The yield % in both cases reached to 60%.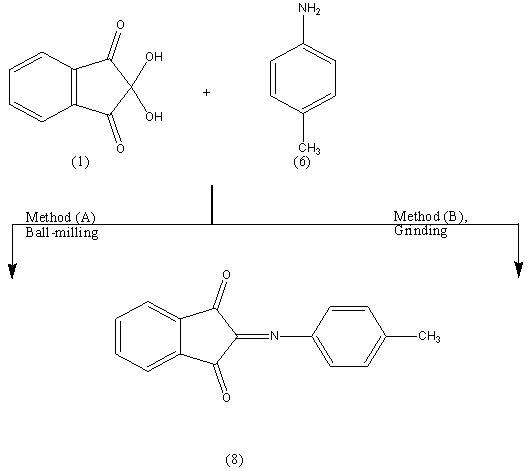 The ball milling reaction of ninhydrin 1 with p-toulidine 6 at room temperature afforded only the corresponding azomethine 8 in a quantitative yield. The water of reaction was easily removed by heating to 80°C in a vacuum. The chemical structure of 8 was established on the basis of spectral data. The IR spectrum confirmed the presence of -C=N and C=O stretching absorption bands at 1592, 1710 cm-1. The mass spectrum showed a molecular ion peak (M+) at m/z = 249.18 (75.2%)(M+), corresponding to the molecular weight of the molecular formula C16H11NO2= 249.18.It has been reported[3,4] that reaction between ninhydrin (1) and malononitrile (9) in clay or H2O yield compound (10) in 80% yield, and we found that reaction between (1) and (9) without the aid of any catalyst and by using ball-milling yields the same product in 100% yield.
The ball milling reaction of ninhydrin 1 with p-toulidine 6 at room temperature afforded only the corresponding azomethine 8 in a quantitative yield. The water of reaction was easily removed by heating to 80°C in a vacuum. The chemical structure of 8 was established on the basis of spectral data. The IR spectrum confirmed the presence of -C=N and C=O stretching absorption bands at 1592, 1710 cm-1. The mass spectrum showed a molecular ion peak (M+) at m/z = 249.18 (75.2%)(M+), corresponding to the molecular weight of the molecular formula C16H11NO2= 249.18.It has been reported[3,4] that reaction between ninhydrin (1) and malononitrile (9) in clay or H2O yield compound (10) in 80% yield, and we found that reaction between (1) and (9) without the aid of any catalyst and by using ball-milling yields the same product in 100% yield.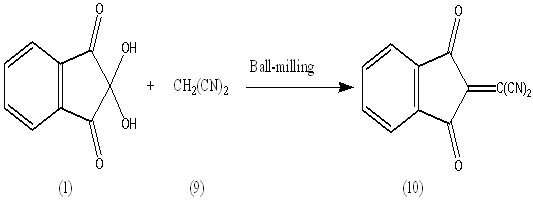 The formation of (10) finds support from its analytical and spectral data; the IR spectrum showed absorption band at (1707 and 2200 cm-1) for C=O and C≡N.The 1HNMR spectrum showed bands at δ 7.4-8.4 ppm for Ar-H and the mass spectrum exhibited the molecular ion peak at m/z= 208.74 [M+] corresponding to the molecular weight of the molecular formula C12H4O2N2 = 208.17By repeating the above reaction in (EtOH/AcOH ratio 7:3) it gives compound (11).
The formation of (10) finds support from its analytical and spectral data; the IR spectrum showed absorption band at (1707 and 2200 cm-1) for C=O and C≡N.The 1HNMR spectrum showed bands at δ 7.4-8.4 ppm for Ar-H and the mass spectrum exhibited the molecular ion peak at m/z= 208.74 [M+] corresponding to the molecular weight of the molecular formula C12H4O2N2 = 208.17By repeating the above reaction in (EtOH/AcOH ratio 7:3) it gives compound (11).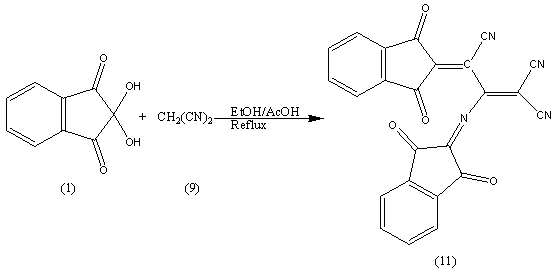 We provides a plausible mechanism;
We provides a plausible mechanism;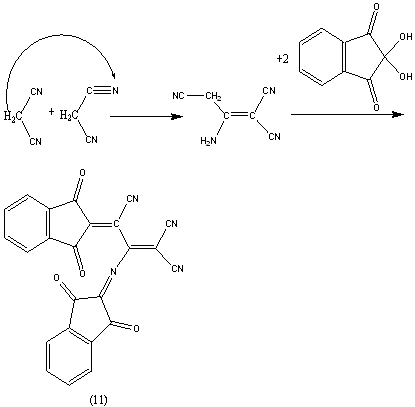 The formation of (11) finds support from its analytical and spectral data; the IR spectrum showed absorption band at (1706 and 2196 cm-1) for C=O and C≡N.The 1HNMR spectrum showed bands at δ 7.5-8.3 ppm for Ar-H and the mass spectrum exhibited the molecular ion peak at m/z= 416.38 [M+] corresponding to the molecular weight of the molecular formula C24H8O4N4 = 416.38.
The formation of (11) finds support from its analytical and spectral data; the IR spectrum showed absorption band at (1706 and 2196 cm-1) for C=O and C≡N.The 1HNMR spectrum showed bands at δ 7.5-8.3 ppm for Ar-H and the mass spectrum exhibited the molecular ion peak at m/z= 416.38 [M+] corresponding to the molecular weight of the molecular formula C24H8O4N4 = 416.38.3. Experimental
- Melting points are uncorrected and were determined with a Fisher-Johns melting point apparatus. Elemental analyses were carried out at Microanalytical Unit of the Faculty of Science, Mansoura University; the results were in satisfactory agreement with the calculated values. IR spectra (KBr) were recorded with 5000 FTIR spectrometer (not all frequencies are reported). The 1H -NMR spectra were acquired using a Bruker WP 300 spectrometer at 300 MHz (1H) or 75.5MHz (13C) in broad band mode. Mass spectra were obtained at a Finnigan MAT 212 instrument by electron impact at 70 eV. The ball-mill was a Retsch MM 2000 swing mill with a 10 cm3 stainless steel, double-walled beaker with fittings for circulating coolants. Two stainless steel balls of 12 mm diameter were used. Ball-milling was performed at 20225 Hz frequency, usually at room temperature (without circulating liquid and the temperature did not rise above 30°C). Reaction of ninhydrin 1 with benzidine 2: Formation of 3Method (A); Ball-milling A mixture of 356 mg of ninhydrin 1 (2.00 mmol) and 184 mg of benzidine 2 (1.00 mmol) was ball-milled at room temperature for 1 h. The material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 3 with 100% yield and did not require purifying workup.Method (B); Using SolventA mixture of 356 mg of ninhydrin 1 (2.00 mmol) and 184 mg of benzidine 2 (1.00 mmol) was refluxed in 10 ml absolute EtOH and 2 ml glacial AcOH for 10 hrs, left to cool. The ppt. formed filtered off and left to dry, recrystallize from EtOH, to give pure 3 with 60% yield. Method (C); By GrindingA mixture of 178 mg of ninhydrin 1 (1.00 mmol) and 184 mg of benzidine 2 (1.00 mmol) was grinded in a mortar at room temperature for 30 min, material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 3 with 60% yield and did not require purifying workup.The product 3Brownish powder mp 300oC; IR (KBr):
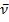 1718 (C=O), 1608 (C=N) cm-1; 1H NMR (200 MHz, [D6] DMSO, 25oC, TMS) δ = 7.50-8.00 (m,7H, ArH) ppm. MS (EI, 70 eV): m/z (%) = 469.04 [M+]. C30H16N2O4 (468.46): calcd. C 76.92 H 3.44 N 5.98 O 13.66; found C 67.93 H 3.40 N 5.95 O 13.72.Reaction of ninhydrin 1 with o-phenylenediamine 4, formation of 5Method (A); Ball-milling A mixture of 178 mg of ninhydrin 1 (1.00 mmol) and 108 mg of o-phenylenediamine 4 (1.00 mmol) was ball-milled at room temperature for 1 h. The material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 5 with 100% yield and did not require purifying workup.Method (B); Using SolventA mixture of 356 mg of ninhydrin 1 (2.00 mmol) and 216 mg of o-phenylenediamine 4 (2.00 mmol) was refluxed in 10 ml absolute EtOH and 2 ml glacial AcOH for 5 hrs, left to cool. The ppt. formed filtered off and left to dry, recrystallize from EtOH, to give pure 5 with 60% yield. The product 5 Green powder mp 227-229oC; IR (KBr):
1718 (C=O), 1608 (C=N) cm-1; 1H NMR (200 MHz, [D6] DMSO, 25oC, TMS) δ = 7.50-8.00 (m,7H, ArH) ppm. MS (EI, 70 eV): m/z (%) = 469.04 [M+]. C30H16N2O4 (468.46): calcd. C 76.92 H 3.44 N 5.98 O 13.66; found C 67.93 H 3.40 N 5.95 O 13.72.Reaction of ninhydrin 1 with o-phenylenediamine 4, formation of 5Method (A); Ball-milling A mixture of 178 mg of ninhydrin 1 (1.00 mmol) and 108 mg of o-phenylenediamine 4 (1.00 mmol) was ball-milled at room temperature for 1 h. The material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 5 with 100% yield and did not require purifying workup.Method (B); Using SolventA mixture of 356 mg of ninhydrin 1 (2.00 mmol) and 216 mg of o-phenylenediamine 4 (2.00 mmol) was refluxed in 10 ml absolute EtOH and 2 ml glacial AcOH for 5 hrs, left to cool. The ppt. formed filtered off and left to dry, recrystallize from EtOH, to give pure 5 with 60% yield. The product 5 Green powder mp 227-229oC; IR (KBr): 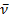 1727 (C=O), 1650 (C=N) cm-1; 1H NMR (200 MHz, CDCl3, 25oC, TMS) δ = 7.50-8.50 (m,8H, ArH) ppm. MS (EI, 70 eV): m/z (%) = 232.33 [M+]. C15H8N2O (232.24): calcd. C 77.58 H 3.47 N 12.06 O 6.89; found C 78.01 H 3.42 N 12.03 O 7.00.Reaction of product 5 with p-toulidine 6: formation of 7A mixture of 696 mg of product 5 (3.00 mmol) and 321 mg of p-toulidine 6 (3.00 mmol) was refluxed in 10 ml absolute EtOH and 2 ml glacial AcOH for 3 hours, left to cool. The ppt. formed filtered odd and left to dry, recrystallize from EtOH, to give pure 7.The product 7Reddish orange powder mp 195oC; IR (KBr):
1727 (C=O), 1650 (C=N) cm-1; 1H NMR (200 MHz, CDCl3, 25oC, TMS) δ = 7.50-8.50 (m,8H, ArH) ppm. MS (EI, 70 eV): m/z (%) = 232.33 [M+]. C15H8N2O (232.24): calcd. C 77.58 H 3.47 N 12.06 O 6.89; found C 78.01 H 3.42 N 12.03 O 7.00.Reaction of product 5 with p-toulidine 6: formation of 7A mixture of 696 mg of product 5 (3.00 mmol) and 321 mg of p-toulidine 6 (3.00 mmol) was refluxed in 10 ml absolute EtOH and 2 ml glacial AcOH for 3 hours, left to cool. The ppt. formed filtered odd and left to dry, recrystallize from EtOH, to give pure 7.The product 7Reddish orange powder mp 195oC; IR (KBr): 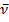 1650 (C=N) cm-1. MS (EI, 70 eV): m/z (%) = 321.43(100%) [M+]. C15H8N2O (321.37): calcd. C 82.22 H 4.70 N 13.08; found C 82.25 H 4.74 N 13.13.Reaction of ninhydrin 1 with p-toulidine 6: Formation of 8Method (A); Ball-milling A mixture of 356 mg of ninhydrin 1 (2.00 mmol) and 214 mg of p-toulidine 6 (2.00 mmol) was ball-milled at room temperature for 1 h. The material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 8 with 100% yield and did not require purifying workup.Method (B); By GrindingA mixture of 356 mg of ninhydrin 1 (2.00 mmol) and 214 mg of p-toulidine 6 (2.00 mmol) was grinded in a mortar at room temperature for 30 min, material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 8 with 60% yield and did not require purifying workup.The product 8Brownish powder mp 85-90oC; IR (KBr):
1650 (C=N) cm-1. MS (EI, 70 eV): m/z (%) = 321.43(100%) [M+]. C15H8N2O (321.37): calcd. C 82.22 H 4.70 N 13.08; found C 82.25 H 4.74 N 13.13.Reaction of ninhydrin 1 with p-toulidine 6: Formation of 8Method (A); Ball-milling A mixture of 356 mg of ninhydrin 1 (2.00 mmol) and 214 mg of p-toulidine 6 (2.00 mmol) was ball-milled at room temperature for 1 h. The material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 8 with 100% yield and did not require purifying workup.Method (B); By GrindingA mixture of 356 mg of ninhydrin 1 (2.00 mmol) and 214 mg of p-toulidine 6 (2.00 mmol) was grinded in a mortar at room temperature for 30 min, material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 8 with 60% yield and did not require purifying workup.The product 8Brownish powder mp 85-90oC; IR (KBr): 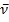 1710 (C=O), 1590 (C=N) cm-1. MS (EI, 70 eV): m/z (%) = 249.07 (61.7%)[M+]. C16H11NO2 (249.26): calcd. C 77.10 H 4.45 N 5.62 O 12.84; found C 77.12 H 4.50 N 5.64 O 12.81.Reaction of ninhydrin 1 with malononitrile 9: Formation of 10Method (A); Ball-milling A mixture of 534 mg of ninhydrin 1 (3.00 mmol) and 198 mg of malononitrile 9 (3.00 mmol) was ball-milled at room temperature for 1 h. The material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 10 with 100% yield and did not require purifying workup.Method (C); By GrindingA mixture of 534 mg of ninhydrin 1 (3.00 mmol) and 198 mg of malononitrile 6 (3.00 mmol) was grinded in a mortar at room temperature for 30 min, material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 10 with 60% yield and did not require purifying workup.The product 10Green powder mp 210-213oC; IR (KBr):
1710 (C=O), 1590 (C=N) cm-1. MS (EI, 70 eV): m/z (%) = 249.07 (61.7%)[M+]. C16H11NO2 (249.26): calcd. C 77.10 H 4.45 N 5.62 O 12.84; found C 77.12 H 4.50 N 5.64 O 12.81.Reaction of ninhydrin 1 with malononitrile 9: Formation of 10Method (A); Ball-milling A mixture of 534 mg of ninhydrin 1 (3.00 mmol) and 198 mg of malononitrile 9 (3.00 mmol) was ball-milled at room temperature for 1 h. The material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 10 with 100% yield and did not require purifying workup.Method (C); By GrindingA mixture of 534 mg of ninhydrin 1 (3.00 mmol) and 198 mg of malononitrile 6 (3.00 mmol) was grinded in a mortar at room temperature for 30 min, material obtained was dried at 0.01 bar at 80 °C in vacuum to give pure 10 with 60% yield and did not require purifying workup.The product 10Green powder mp 210-213oC; IR (KBr): 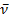 1707 (C=O), 2229, 2200 (C≡N) cm-1; 1H NMR (200 MHz, CDCl3, 25oC, TMS) δ = 7.50-8.50 (m,8H, ArH) ppm. MS (EI, 70 eV): m/z (%) = 208.74 [M+]. C12H4N2O2 (208.17): calcd. C 69.24 H 1.94 N 13.46 O 15.37; found C 69.26 H 1.93 N 13.51 O 15.40.Method (B); Using SolventA mixture of 891 mg of ninhydrin 1 (5.00 mmol) and 990 mg of malononitrile 6 (5.00 mmol) was refluxed in 10 ml absolute EtOH and 2 ml glacial AcOH for 8 hrs, left to cool. The ppt. formed filtered off and left to dry, recrystallize from EtOH, to give pure 11 with 60% yield. The product 11Greenish powder mp 243-245oC; IR (KBr):
1707 (C=O), 2229, 2200 (C≡N) cm-1; 1H NMR (200 MHz, CDCl3, 25oC, TMS) δ = 7.50-8.50 (m,8H, ArH) ppm. MS (EI, 70 eV): m/z (%) = 208.74 [M+]. C12H4N2O2 (208.17): calcd. C 69.24 H 1.94 N 13.46 O 15.37; found C 69.26 H 1.93 N 13.51 O 15.40.Method (B); Using SolventA mixture of 891 mg of ninhydrin 1 (5.00 mmol) and 990 mg of malononitrile 6 (5.00 mmol) was refluxed in 10 ml absolute EtOH and 2 ml glacial AcOH for 8 hrs, left to cool. The ppt. formed filtered off and left to dry, recrystallize from EtOH, to give pure 11 with 60% yield. The product 11Greenish powder mp 243-245oC; IR (KBr):  1633 (C=N), 1672,1706,1745,1767 (C=O), 2196,2217 (C≡N) cm-1; 1H NMR (200 MHz, CDCl3, 25oC, TMS) δ = 7.50-8.50 (m,8H, ArH) ppm. MS (EI, 70 eV): m/z (%) =416.38 [M+]. C24H8N4O4 (416.34): calcd. C 69.24 H 1.94 N 13.46 O 15.37; foun
1633 (C=N), 1672,1706,1745,1767 (C=O), 2196,2217 (C≡N) cm-1; 1H NMR (200 MHz, CDCl3, 25oC, TMS) δ = 7.50-8.50 (m,8H, ArH) ppm. MS (EI, 70 eV): m/z (%) =416.38 [M+]. C24H8N4O4 (416.34): calcd. C 69.24 H 1.94 N 13.46 O 15.37; foun Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML