-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2011; 1(1): 6-9
doi: 10.5923/j.ajoc.20110101.02
Acid Catalysed Isomerization of Nimbin to Isonimbin
S. Narasimhan 1, R. Mohankumar 1, V. P. Santhanakrishnan 2, V. Radhakrishnan 1
1Asthagiri Herbal Research Foundation, 162A-Perungudi Industrial Estate, Perungudi, 600096, Chennai
2Department of Biotechnology, Centre for Plant Molecular Biology & Biotechnology, Tamil Nadu Agricultural University, 641003, Coimbatore
Correspondence to: S. Narasimhan , Asthagiri Herbal Research Foundation, 162A-Perungudi Industrial Estate, Perungudi, 600096, Chennai.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The tetranortriterpenoid, nimbin contains the olefinic double bond at the ring C. When Ritter reaction was performed on this compound to introduce the amide moiety in ring C using sulfuric acid in acetonitrile, unexpectedly it underwent isomerization with the formation of isonimbin.
Keywords: Nimbin, Isonimbin, Acetonitrile, Sulphuric Acid, Neemoil
Cite this paper: S. Narasimhan , R. Mohankumar , V. P. Santhanakrishnan , V. Radhakrishnan , "Acid Catalysed Isomerization of Nimbin to Isonimbin", American Journal of Organic Chemistry, Vol. 1 No. 1, 2011, pp. 6-9. doi: 10.5923/j.ajoc.20110101.02.
Article Outline
1. Introduction
- Nimbin, a C-seco limonoid of biological significance present in the seed oil of Azadirachta indica A.juss 1,2. has got diversified functional groups in the structure and has shown substantial promise in insect control3,4. Literature reports that photo-oxidised products of this compound showed a marked increase in the antifeedant activity than compared to the parent compound5,6 which gave impetus for performing the semi synthetic modification of the compound to introduce the nitrogen functionality through C-N bond formation. Hence Ritter reaction7-10 was attempted to introduce the amide group across the olefinic double bound in ring C in nimbin.Thus nimbin treated with acetonitrile in sulfuric acid at 0℃ resulted in the formation of the product whose 1H-NMR and 13C-NMR does not contain the peak corresponding to the amide moiety and the number of the carbons in both the starting material and the product remained the same indicating a rearrangement reaction rather than the Ritter reaction.
2. Results and Discussion
- A comparative analysis of the 1H & 13C NMR spectra (Figure.4.3a & 4.3b) of the substrate11 and product revealed the characteristic signals corresponding to enone (6.34 & 5.85ppm in 1H NMR & 147.59 & 125.99ppm in 13C NMR), furan ring (7.32, 6.45 & 7.59ppm in 1H NMR & 119.19, 109.35, 139.52 & 142.47ppm in 13C NMR). The basic skeleton of the product is same as in the substrate except for changes in the D–ring.The DEPT-135 spectrum of the compound showed 30 carbon signals, which are the same as in the starting material. The 30 carbons consisted of 10 quaternary, 11 methine, 2 methylene and 7 methyl. The functional groups like enone, ester and acetate were intact. The number of quaternary, methylene, methine and methyl carbons remain unaltered. But the oxygenated carbon signal at 86.97ppm corresponding to C-15 is shifted from a methine environment to quaternary carbon, which is also evident from the disappearance of 1H NMR signal at 5.57ppm corresponding to H-15. This suggested a probable D-ring opening and closure. This has resulted in a similar rearrangement12 observed in few other limonoids such as nimbolide, salannin etc. There is a shift in C-13 signal from 135.01 to 94.83ppm, C-14 signal from 145.98 to 55.91ppm, C-15 from 86.97 to 31.29ppm, C-16 from 41.46 to 126.20ppm and C-17 from 49.33 to 137.09ppm. The new signal at 94.83ppm can be attributed to the oxygen-linked carbon. The rearrangement has resulted in appearance of multiplets at 2.34 and 5.74ppm corresponding to the H-15 & H-16, which originally appeared at 5.57 and 2.19ppm in the substrate. From the above discussion it is evident that there exists a possibility of double bond isomerization. The structure of the product is given in the Scheme 1. Isonimbin has a molecular formula (C30H36O9). UV spectrum of the compound showed λmax at 226 nm. Melting point: 212-215℃. A complete assignment of the proton and carbon signals in the spectrum of isonimbin is represented in the experimental session.
3. Possible Mechanism for Rearrangement of Nimbin to Isonimbin
- Attempts to introduce C-N bond formation (Ritter reaction) resulted in novel rearranged product. The mechanism involves H+ ion coordination with etheral oxygen of the C-ring followed by cleavage resulting to form a more stable allyl carbocation at the C-13 and deprotonation of H-17 results in a diene. This diene undergoes a 1,5 sigmatropic shift of the hydrogen atom followed by protonation at the C-14 position.This results in a stable allyl carbocation, at the C-13 position and migration of the double bond between C-16 & C-17. Successive intramolecular attack of the H+ on the tertiary carbocation results in 180o rotation of bond between C-8 & C-14 and subsequent ring closure to produce the rearranged product with intact cyclic ether namely isonimbin.
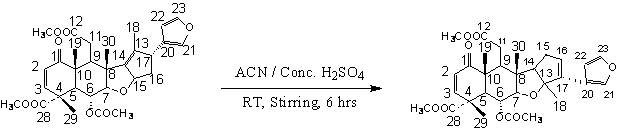 | Scheme 1. Acid catalysed isomerization of the nimbin. |
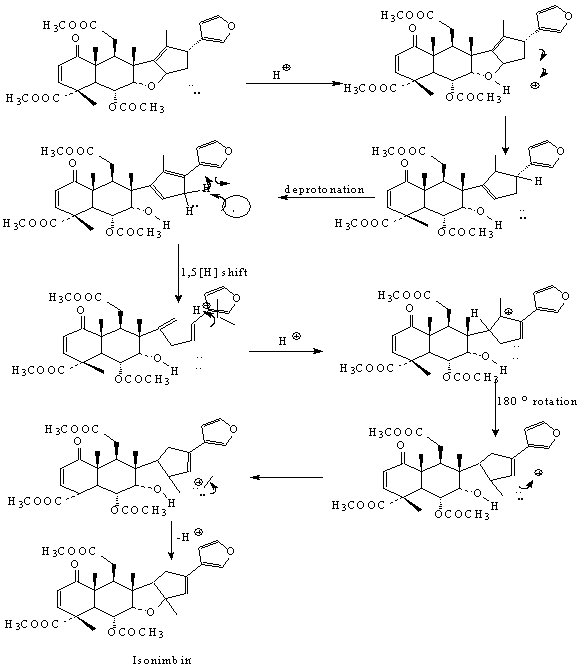 | Scheme 2. possible mechanism for the isomerization of Nimbin. |
|
4. Experimental
- NMR spectrum was recorded on a Bruker 500MHz instrument using TMS as an internal standard and CDCl3 as the solvent. HPLC was performed on Shimadzu instrument with LC-10ATVP high pressure pump and C18 reverse phase Luna 5u column (250 x 4.60mm) and the peaks detected at 215nm (SPD-10 AVP UV-VIS Detector) and the mobile phase being acetonitrile: water (60:40) at a flow rate of 0.5ml/min. Mass spectrum was recorded on a Shimadzu QP 1000A and QP 5000 mass spectrometer. Melting point was determined using a Raaga industries melting point apparatus and is uncorrected.
4.1. Isolation of Nimbin
- Neem Oil (50g) was dissolved in methanol: water (60:40, 500ml) and partitioned with chloroform (200ml x 3) and chloroform removed using rotary evaporator under vacuum. The above chloroform extract (38g) was admixed with 80g of silica gel (70-325 mesh), dried and admixture loaded in a column packed with silica gel using hexane as solvent. Initially the column was eluted with hexane followed by increasing order of polarity with ethylacetate and Nimbin eluted at 18% ethylacetate in hexane and compared well with standard nimbin in TLC, the fraction with similar Rf were pooled and solvent removed to yield pure Nimbin (680mg, 0.014%).
4.2. Modification of Nimbin to Isonimbin
- Nimbin (0.5mmol) was taken in a 100ml single necked round bottom flask fitted with guard tube, followed by 10ml of acetonitrile. The contents were cooled to 0oC and 0.5ml of concentrated sulphuric acid added slowly with stirring. The reaction was left to attain room temperature and additionally stirred for 6hrs. The completion of the reaction was monitored by TLC. After completion, the flask was immersed in ice-bath and aqueous ammonia was added slowly untill the pH is 7-8, the solution was concentrated under reduced pressure using rotary evaporator and poured into ice-cold water and extracted using ethyl acetate and the crude extract was purified using flash (under nitrogen) column chromatography and pure product eluted at 16% Ethyl acetate in hexane (Isonimbin - Yield: 62%).
5. Supporting Information
- 1H NMR (500MHz, CDCl3,): δ = 0.96 (s, 3H, H-30), 1.29 (s, 3H, H-29), 1.34 (s, 3H, H-19), 1.66 (s, 3H, H-18), 1.90 (s, 3H, OCOCH3), 2.13 & 2.70 (m, 2H, H-11a & 11b), 2.34 (m, 2H, H-15), 2.50 (m, 1H, H-14), 2.70 (m, 1H, H-9), 3.50 (d, 1H, J = 3.0Hz, H-5), 3.76 (s, 3H, -COOCH3), 3.80 (d, 1H, J = 12.3Hz, H-7), 3.81 (s, 3H, -COOCH3), 5.19 (dd, 1H, J = 3.0, 12.3Hz, H-6), 5.74 (m, 1H, H-16), 5.85 (d, 1H, J = 10.5Hz, H-2), 6.34 (d, 1H, J = 10.4Hz, H-3), 6.45 (m, 1H, H-22), 7.32 (m, 1H, H-21), 7.59 (m, 1H, H-23).13C NMR (500MHz, CDCl3): δ = 16.67 (C-30), 16.96 (C-19,17.1 (C-29), 20.62 (OCOCH3),24.2 (C-18), 31.3(C-15), 33.2 (C-11), 41.3 (C-9), 42.3 (C-5), 47.0 (C-4),48.9 (C-10), 49.9 (C-8), 51.8 (-COOCH3), 52.7 (-COOCH3), 55.9 (C-14), 68.3 (C-6), 79.9 (C-7) ,94.8 (C-13), 109.4 (C-22), 119.2 (C-20), 126.0 (C-2), 126.2 (C-16), 137.1 (C-17), 139.5 (C-21), 142.5 (C-23), 147.6 (C-3), 170.3 (OCOCH3), 174.9 (C-12), 174.9 (C-28), 201.8(C-1).
6. Conclusions
- Thus the attempts to introduce the nitrogen in nimbin by Ritter reaction has resulted in an unexpected rearranged product isonimbin.
ACKNOWLEDGEMENTS
- RMK thanks DBT, VPS and VRK thank CSIR for funding support and Spic Science Foundation for the Analytical facility.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML