-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Organic Chemistry
p-ISSN: 2163-1271 e-ISSN: 2163-1301
2011; 1(1): 1-5
doi: 10.5923/j.ajoc.20110101.01
Terpenoids of the Heartwood of Chamaecyparis Nootkatensis
M. A. Khasawneh 1, J. J. Karchesy 2
1Department of Chemistry, Faculty of Science, United Arab Emirates University, Al-Ain, P.O. Box: 17551, U.A.E
2Department of Forest Products and Engineering, College of Forestry, Oregon State University, Corvallis, Oregon 97330, U.S.A
Correspondence to: J. J. Karchesy , Department of Forest Products and Engineering, College of Forestry, Oregon State University, Corvallis, Oregon 97330, U.S.A.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Two monoterpenes and two sesquiterpene diols were isolated and identified for the first time from the methanol extract of the heartwood of Alaska yellow cedar (AYC) tree. The two monoterpenes were identified as (1S)-2-oxo-3-p-menthenol (1) and (4R)-4-hydroxy-4-isopropylcyclohex-1-enecarboxylic acid (2). The two sesquiterpenes were identified as (5S, 7R, 10R, 11R)-eudesm-4(14)-ene-11, 12-diol (kudtdiol, 3) and (4R, 5S, 7R)-1(10)-eremophilene- 11,12-diol (tedonodiol, 4). Structures for these compounds were confirmed on the basis of spectroscopic data and in comparison with related known compounds. In addition, 10 other previously known AYC constituents were also isolated from the methanol extract of the heartwood of AYC including nootkatin, chamic acid, chaminic acid, carvacrol, methyl carvacrol, chanootin, nootkatene, nootkatone, valencene and valencene-13-ol.
Keywords: Alaska Yellow Cedar, Natural Products, Tedonodiol, Kudtdiol
Cite this paper: M. A. Khasawneh , J. J. Karchesy , "Terpenoids of the Heartwood of Chamaecyparis Nootkatensis", American Journal of Organic Chemistry, Vol. 1 No. 1, 2011, pp. 1-5. doi: 10.5923/j.ajoc.20110101.01.
Article Outline
1. Introduction
- Alaska yellow cedar (Chamaecyparis nootkatensis (D. Don) spach), also known as yellow cedar, Alaska cedar, yellow cypress, is a medium-sized tree that is up to 25 meters tall and 90 cm in diameter. It is one of the eleven genera that make the Cupressaceae (Cypress) family. The genus Chamaecyparis includes, in addition to Chamaecyparis nootkatensis, other common species such as Chamaecyparis obtusa (Japanese cypress), Chamaecyparis lawsoniana (Port-Orford cedar), Chamaecyparis formosensis, Chamaecyparis thyoides (Atlantic white cedar), Chamaecyparis taiwanensis, etc. Several compounds have been isolated and identified from the heartwood extract of AYC including two tropolone sesquiterpenoids- nootkatin[1,2] and chanootin[3]; four monoterpenoids- chamic acid, chaminic acid, carvacrol, methyl carvacrol[4,5]; and four eremophilane-type sesquiterpenoids- nootkatene, nootkatone, valencene[6,7] and valencene-13-ol[8]. This paper describes the isolation and characterization of two monoterpenoids (1, 2) and sesquiterpenoids (3, 4) from the methanolic extract of AYC heartwood (Table 1).
2. Results and Discussion
2.1. (-)-(1S)-2-Oxo-3-P-Menthenol, (1)
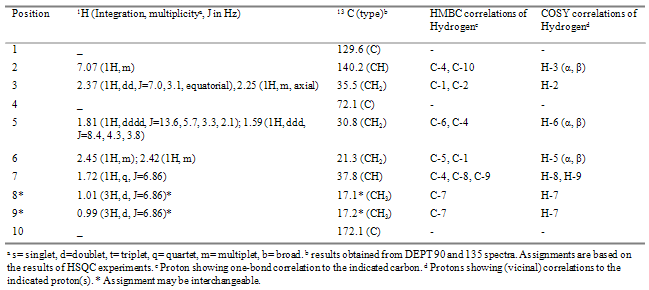
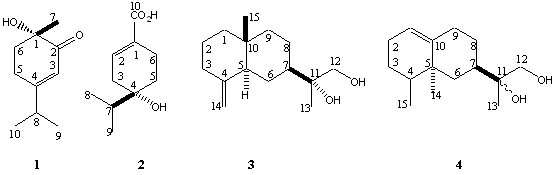
- This compound was isolated as yellow oil; [α]D20 - 85º (c 0.455, CHCl3); IR (thin film) νmax 3450, 1715, 1620cm-1; EIMS m/z [M]+ 168, 153, 151, 125; HRCIMS m/z [M]+ 168.1143, calculated for C10H16O2, requires 168.1150. NMR spectral data are in Table 1. IR spectrum of 1 showed a strong absorption band at 1717cm-1 and 3450cm-1 characteristic of carbonyl and hydroxyl groups, respectively. The presence of two closely similar methyl groups that exhibit doublet signals suggests that there is an isopropyl group in this compound. The third methyl group being a singlet and shifted downfield suggests that it is attached to the ring where a hydroxyl group is also attached. The carbon atom NMR signal at 172.8ppm is generally of higher value than expected for alkenes, but a closer look at the literature reveals that high values like what we have here are observed for α,β-unsaturated carbonyl compounds. Further support for this conclusion comes from the 13C-NMR signal at 203ppm for the carbonyl carbon. Further details of structure 1 were verified by 2-D NMR spectra including HMBC and COSY (Table 1). These results reveal that the structure follows the menthane-type skeleton pattern frequently observed in plant species. Thedownfield -shifted methyl group (at δH 1.33) is located at C-1 as evidenced by the HMBC correlations of its protons with C-2, C-6 and C-1 (203.3, 36.1 and 73.1ppm, respectively). The absence of correlations between the vinylic proton and the carbonyl carbon from one side and that between proton at δH 2.48 with the same carbon support the earlier conclusion that the isopropyl group is located at the β–carbon. Key COSY correlations of H-5 with H-6 and H-8 with H-9 and H-10 add further support for structure 1.The absolute stereochemistry around C-1 of this compound is extremely difficult to establish because it is a tertiary alcohol. However, 1 is related to a compound previously described in the literature as a product of the microbial fermentation of α–terpinene. Compound 1 gave essentially identical 1H and 13C NMR data as one of these products, which is (+)-(1R)-2-oxo-3-p-menthenol). However, optical rotation data obtained for 1 suggest that it is the enantiomer of the compound described in the literature (1 gave [α]D - 85º (c 0.455, CHCl3), while the literature compound gave [α]D + 92.9 (c 1.00, CHCl3,[9]. Based on this notion, the absolute configuration around C-1 in compound 1 was indirectly deduced to be “S”. Compound 1 was also reported by [10] as a chemical oxidation product of α–terpinene. However, only IR data were reported in this reference.
2.2. (+)-(4R)-4-Hydroxy-4-Isopropyl-Cyclohex-1- Enecarboxylic Acid, 2
- This compound was isolated as white amorphous solid; [α]D20 + 25.5 (c 0.275, CHCl3); IR (thin film) νmax 2500-3500, 1620cm-1; EIMS m/z 184 [M+], 167 [184-OH], 166 [M+-H2O], 151 [166-Me], 141 [[M+-CH3CHCH3], 138 [166-CO], 123 [138-Me]; HRCIMS [M]+ 184.1088 calculated for C10H16O3, requires 184.10995. NMR spectral data are in Table 2. From the molecular formula of C10H16O3 it follows that there are three degrees of un-saturation in this compound and from NMR data (both 1H and 13C), there is only one C-C double bond. IR spectrum showed a strong absorption band at 2500-3500cm-1 characteristic of a carboxylic acid, which accounts for another degree of un-saturation. Therefore, there must be a ring in the structure of this molecule. The presence of two closely similar methyl groups in the 1H NMR spectrum that exhibit doublet signals suggests that there is an isopropyl group. This conclusion is supported by the presence of a single proton that has a quartet signal correlated with two methyl groups in the COSY (Table 2). Therefore, it can be deduced that the ring is six-membered ring based on simple atom counts. The proton signal at 7.07ppm is generally of higher value than expected for vinylic protons, but a closer look at literature reveals that high values like what we have here are observed for β- protons when there is an electron withdrawing group such as the carboxyl group attached at the α-position of the alkene. Finally, the isopropyl group is deduced to be connected to the ring through C-4 based on HMBC correlations of this carbon with H-8, H-9 and H-7 protons. Other key HMBC correlations include those of H-2 with C-10 and C-4, H-3 with C-2, H-5 with C-4 and C-6 (Table 2). The specific rotation ([α]D20 ) value for compound 2 was measured to be + 25.5 (c 0.275, CHCl3). The absolute stereochemistry was indirectly deduced based on comparison of this compound with another compound reported in the literature as a microbial fermentation product of (-)-β-pinene by Soil Pseudomonad (PL-Strain) [11]. Compound 2 produced an identical 1H NMR spectrum to literature compound. However, these two compounds have opposite optical rotation signs and therefore compound 2 was assigned the "R" stereochemistry at C-4.
|
|
|
2.3. (5S, 7R, 10R, 11R)-Eudesm-4(14)-Ene-11, 12-Diol (Kudntdiol), 3
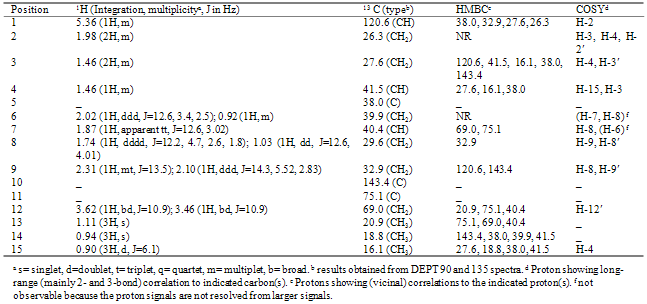
- This compound was isolated as white amorphous solid; [α]D20 +66.8 (c 0.25, CHCl3); IR (thin film) νmax 3450cm-1; EIMS m/z [M]+ 238 (M+), 220 (M+-H2O), 207 (M+-CH2OH), 202 (M+-2H2O) and 189 (M+-CH2OH-OH); HRCIMS m/z [M]+ 238.19377, calculated for C15H26O2, requires 238.1933. 1H NMR spectral data were in good agreement with literature data for kudntdiol[12] and therefore are not listed here. The value of the optical rotation for kudtdiol isolated in this work from AYC ([α]D +66.8 (c 0.25, CHCl3)) is in good agreement with the reported value for the same compound in the literature([α]D +72.9 (CHCl3)). The absolute stereochemistry around the bicyclic ring chirality centers (C-5, C-7, C-10, and C-11) of 3 was established in reference [13]. Compound 3 belongs to a family of sesquiterpenes called the eudesmanes, which is a large group of sesquiterpenes widely occurring in the plant kingdom. It has been previously isolated from the aerial parts of Jasonia glutinosa and named as kudtdiol[13] and it has not been reported for any other species to date. Therefore, the isolation of kudtdiol (3) from AYC is a new and important finding. This is in fact the first report to indicate the presence of a eudesmane-type skeleton in AYC. This fact makes AYC a unique species in the Cupressaceae family since the eremohpilane-type sesquiterpenes are extremely rare in this family. Chamaecyparis lawsoniana (Port Orford Cedar) on the other hand, has been shown to have eudesmane-type skeleton as the dominant sesquiterpene content.
2.4. (4R, 5S, 7R)-1(10)-Eremohpilen-11, 12-Diol, 4
- This compound was isolated as yellow oil; [α]D20 -17.3 (c 0.66, CHCl3); IR (thin film) νmax 3450, 1620cm-1; EIMS m/z 238 (M+), 220 (M+-H2O), 207 (M+-CH2OH), 202 (M+-2H2O), 189(M+-(CH2OH+OH), 161 (189-CHCH3, the decaline system residue); HRCIMS m/z [M]+ 238.1938, calculated for C15H26O2, requires 238.1933). NMR spectral data are included in Table 3. These data were in agreement with those reported by (Guerreiro et al 1979). The molecular formula was determined on the basis of HRCIMS ion peak at 238.1938 to be C15H26O2 (required for C15H26O2 is 238.1933). EIMS showed peaks at 238 (M+), 220 (M+-H2O), 207 (M+-CH2OH), 202 (M+-2H2O), 189(M+-(CH2OH+OH), 161 (189-CHCH3, the decaline system residue) as the main fragment peaks. IR spectroscopy indicated the presence of an alcoholic functional group in the molecule.All the above results suggest that the structure of this compound maybe related to that of 3 described above. However, a closer look at the 2-D NMR (HMBC and COSY), reveals that the structure of 4 exhibits the eremophilane skeleton, which is widely found in the heartwood of AYC. Among the key correlations observed in the HMBC spectrum, which played a major role in the assignment of the structure of this compound are the H-14 correlations with C-10, C-4 and C-6. These correlations show that this methyl is attached to C-5. Other important correlations are those of H-15 with C-14, C-3, C-4 and C-5, which suggests that the C-15 methyl is attached to C-4 and that C-15 and C-14 methyls are vicinal to each other. Correlations of H-12 and H-13 with C-7 on one hand and those of H-7 with C-11, C-12 and C-13 on the other hand demonstrate that the attachment between the bicyclic ring and the side chain occurs as expected, through C-7. HMBC correlations of H-12 with C-11 and C-13 and correlations of H-13 with C-11 and C-12 suggest that the side chain diol has the arrangement shown in the structure. COSY spectrum correlations provide a support for these conclusions.Compound 4 was previously isolated as a natural product from the plant Tessaria dodonelfolia and named as tedonodiol[14]. The structure was assigned mainly based on chemical transformations as well as 1H NMR data, of which only the most important part was described while no 13C-NMR data were reported. A comparison of the data reported in the present work and those in the reference reveals that there is good agreement in the 1H NMR data except that there is a systematic shift of about positive 0.07-0.10 δH units in the data reported here for most of the signals. The value of the optical rotation was measured to be [α]589 –17.3 (c 0.66, CHCl3)[14]. To shed some light on the conformational arrangement of the bicyclic system of 4, the nuclear overhauser effect (NOE) should be useful. In the NOESY spectrum measured in this study, the correlation between H-7 and H-14 suggests that these two types of protons occur in spatial proximity (Figure 1). The correlation of H-14 with H-15, which is more difficult but can be observed, suggests that 4 has the usual conformation of eremophilanes isolated previously from AYC.
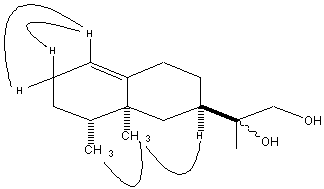 | Figure 1. NOE correlations observed for 4 |
3. Materials and Methods
3.1. Materials
- Hexane (EM Science, Gibbstown, NJ, glass distilled 99.4%) was used as received. Acetone (99.6%), Ethyl Acetate (99.9%), Dichloromethane (99.9%) and Methanol (99.9%) were all purchased from Fisher Scientific, Fair Loan, NJ and distilled prior to use. Diethyl ether (EM Science, Gibbstown, NJ 98%) was used as received. Chloroform (Mallinckrodt Baker Inc., Paris, KY, 99.9%) was used as received. Deuterated chloroform (Aldrich Chemical company, Milwaukee, WI, 99.9 atom %D) was used as received for NMR experiments. Anhydrous Na2SO4 (Fisher Scientific, Fair Lawn, NJ, 100%), Silica gel (EM Science, particle size 0.063-0.200mm, 70-230 mesh ASTM), TLC plates (EM Science, Kieselgel 60 F254 coated on Aluminum support) were all used as received.
3.2. Plant Extraction
- AYC tree was collected from the Hungry Mountain area on the Sol Duc River Drainage on the western slopes of the Olympic Mountains in the Olympic National Forest, WA, USA. A collection permit was obtained from the Sol Duc ranger station in Forks, WA. A botanical voucher specimen (#188046) is deposited in the Oregon State University Herbarium. Heartwood was separated from the rest of the components and chipped in a grinder to a size of approximately 2X1cm chips and kept at room temperature in the dark until used. The chipped AYC heartwood sample (1000g) was soaked in methanol (2.5L) in a 5.0L conical flask with continuous stirring for 24 h. The mixture was gravity filtered through a 15cm filter paper. Anhydrous Na2SO4 was added to the filtrate and the mixture was re-filtered. The filtrate was concentrated in vacuum to give thick dark brown oil (43g, 4.3% based on the wet wood mass).
3.3. Chromatographic Separation of Constituents
- A 1-g sample of the methanol extract was chromatographed on a silica gel column using a gradient elution system of increasing polarity from 100:0 to 50:50 Hex:EtOAc (v/v) resulting in nine main fractions I-VII: fraction I (mainly valencene and nootkatene as checked by TLC and GC against standards), fraction II (mainly carvacrol by TLC and GC), fraction III (mainly nootkatol and nootkatone). Fraction IV was carefully chromatographed over a silica gel column with Hex-EtOAc (80:20) to provide compound 1 (12mg, Rf=0.56, Hex-EtOAc (50:50)) as a colorless oily residue and compound 3 (11mg, Rf=0.46, Hex:EtOAc (50:50)) as a colorless amorphous solid. Fraction V was mainly composed of compound 4 (43mg, Rf=0.36 50:50 Hex:EA) as yellow oil with fair purity (>90%) by NMR. Fraction VI contained mainly chamic acid (13mg) and chaminic acid (8mg) both with good purity (checked by TLC and GC). This fraction was not investigated any further since these two compounds have been described before. Fraction VII contains mainly compound 2 (7mg, Rf=0.24 in Hex-EtOAc (50:50)) with purity of more than 95% shown by NMR. NMR measurements were recorded on a Bruker Avance NMR (400MHz in the case of 1H and 100 MHz in the case of 13C) spectroscopy instrument using CDCl3 as a solvent.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML