-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(10): 3421-3425
doi:10.5923/j.ajmms.20251510.30
Received: Sep. 22, 2025; Accepted: Oct. 12, 2025; Published: Oct. 15, 2025

Immunohistochemical Characteristics of Processes Occurring in Necrotizing Enterocolitis in Neonates
Avaz Azizovich Djurabayev1, Jamoliddin Turgunbaevich Mamasaidov2
1Assistant, Department of Anatomy, Fergana Medical Institute of Public Health, Fergana City, Republic of Uzbekistan
2Head of Department of the Pharmacology, Fergana Medical Institute of Public Health, Fergana City, Republic of Uzbekistan
Correspondence to: Avaz Azizovich Djurabayev, Assistant, Department of Anatomy, Fergana Medical Institute of Public Health, Fergana City, Republic of Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The specific features of immunohistochemical changes arising in neonatal necrotizing enterocolitis (NEC) were investigated with a focus on whether there is a response in the mucosa-associated lymphoid tissue (MALT) that contains immunocompetent cells, and on how the key links in the development of humoral and cellular immunodeficiency form. These were studied in direct relation to the dynamics of postnatal development in newborns. In the 0–7-day period, when MALT structures are still immature, intestinal lesions proceed predominantly with necrosis rather than with pronounced injury to MALT. In the 8–28-day period, the sharp response of relatively more mature immune cells leads to hyperplasia of the mucosa, submucosa, and MALT structures. This confirms that the process takes on a hyperergic character and continues with predominance of a systemic vascular response.
Keywords: Morphometry, Necrotizing enterocolitis, Neonate, Necrosis, Small intestine
Cite this paper: Avaz Azizovich Djurabayev, Jamoliddin Turgunbaevich Mamasaidov, Immunohistochemical Characteristics of Processes Occurring in Necrotizing Enterocolitis in Neonates, American Journal of Medicine and Medical Sciences, Vol. 15 No. 10, 2025, pp. 3421-3425. doi: 10.5923/j.ajmms.20251510.30.
Article Outline
1. Introduction
- Globally, delay in the development of the immune system in newborns contributes to the occurrence of NEC, with an incidence of 20–30 per 1000 neonates. In the USA and Europe, NEC develops in 8–10 per 1000 live births; in the Russian Federation, 14–16 per 1000; and in Central Asian countries, 15–21 per 1000. In neonates, NEC typically manifests with secondary infection and sepsis, and the mortality rate averages 65–71% [1,2,3,4,5,6].The high incidence of NEC in newborns is associated with immaturity of various systems, primarily the immune system. The main precipitating factors for NEC development in neonates are maternal infectious diseases and placental inflammation [8,9,10,11,12,13].As a practical approach to this problem, modern neonatologists consider the implementation of diagnostic and therapeutic methods based on the pathomorphological changes of the small intestine in preterm neonates to be one of the urgent priorities. Preterm infants with NEC exhibit specific clinical-laboratory patterns whose nature is determined by gestational age (Dorofeeva A.D., 2020). According to various authors, the incidence of NEC in newborns ranges from 0.3 to 3.0 per 1000 infants. Among preterm infants and those with intrauterine growth restriction, mortality increases markedly and varies from 28.0% to 54.0%. After surgical interventions, mortality reaches 60.0–100%, which is especially critical for infants with very low birth weight and gestational age under 30 weeks [14-21].
2. Purpose of the Study
- To investigate and refine the immunohistochemical characteristics of the small intestine in the development of necrotizing enterocolitis in neonates.
3. Materials and Methods
- At the Fergana Regional Bureau of Pathological Anatomy (2012–2023), a total of 74 neonates were studied, including 18 controls (newborns who died from cerebral cephalohematoma). Case histories, clinical-anamnestic data, and small-intestinal tissue obtained during autopsy examinations were analyzed. Morphological and immunohistochemical methods were applied to small-intestinal tissue in NEC.
4. Results and Discussion
- This study examined the specificity of conducting immunohistochemical analyses in organized lymphoid tissue of the ileum (one of the peripheral lymphoid organs) and analyzed the morphofunctional changes of ileal lymphoid tissue in the intestinal form of neonatal sepsis. The findings were primarily dependent on the degree of maturation of this tissue, the filling of all morphofunctional areas with characteristic lymphocytes, and the activity of reticular cells and macrophages.CD3 marker. CD3 is a multiprotein complex on lymphocyte membranes that ensures coreceptor binding and is used as a defining marker of T lymphocytes. Low expression of CD3 indicates a sharp decrease of thymic cortical T lymphocytes, most of which have undergone induced apoptosis. Clinico-morphologically, low CD3 expression in neonates with advanced NEC confirms that T-cellular immunity is not fully developed; CD3+ T lymphocytes have not reached secondary peripheral immune organs, T-cell areas have not formed in peripheral lymphoid organs, and T cells have not participated in the infectious process.CD4 marker. CD4 is a monomeric transmembrane glycoprotein of the immunoglobulin superfamily expressed on lymphocyte membranes, predominantly found in marginal zones of intestinal MALT structures. It is used to identify T helpers. CD4 plays an important role in antigen recognition and possesses the ability to bind to membrane coreceptors of T-killers within the marginal zone of MALT. In ~ 65% of cases it is expressed mainly on T helpers and, to a lesser extent, on macrophages, monocytes, and dendritic cells. Low CD4 expression is primarily explained by a sharp reduction in T helpers and by cellular immune deficiency. In our study, low positive CD4 expression in NEC indicates that T helpers act as the primary link enabling lymphocyte subpopulations to function-recognizing antigen and initiating (or failing to initiate) the acquired immune response.
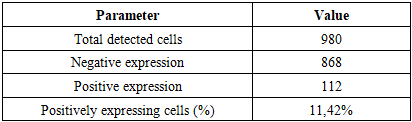 In both the 0–7-day and 8–28-day NEC periods, the lack of distinct T-lymphocyte subpopulations in intestinal MALT was reflected by low positive immunohistochemical expression, confirming that in neonates-with a still immature cellular immune system-NEC in the small intestine proceeds with profound dysregulation.
In both the 0–7-day and 8–28-day NEC periods, the lack of distinct T-lymphocyte subpopulations in intestinal MALT was reflected by low positive immunohistochemical expression, confirming that in neonates-with a still immature cellular immune system-NEC in the small intestine proceeds with profound dysregulation.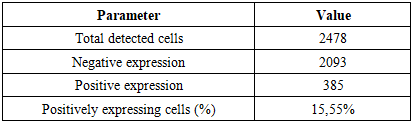
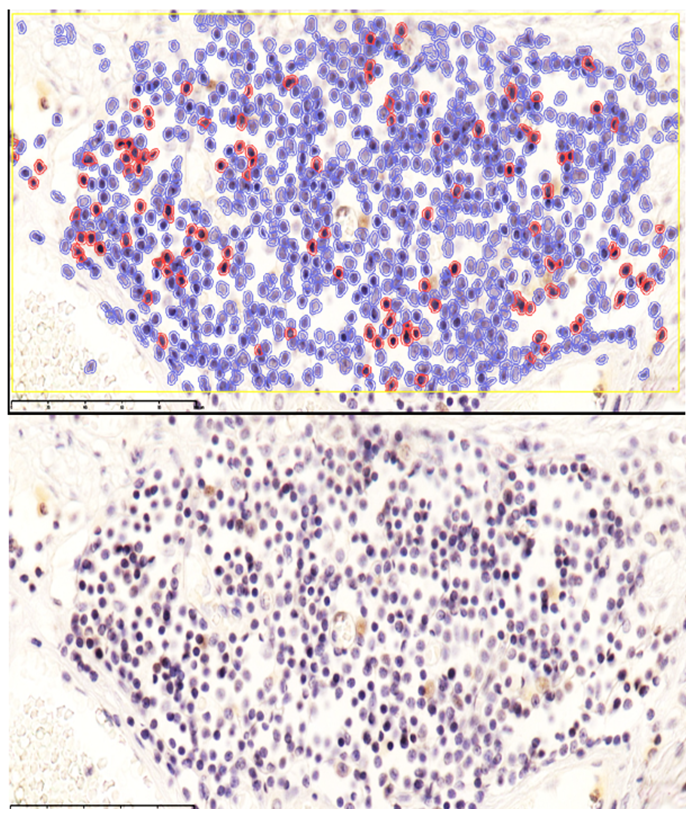
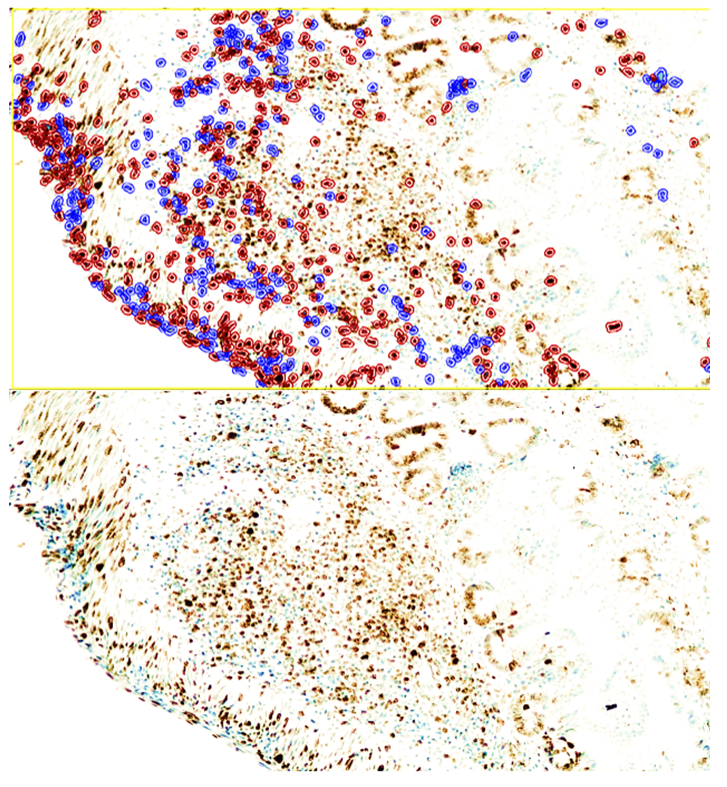 | Figure 3. During days 8–28 of NEC, low positive CD4 reaction in MALT with reduced lymphocytes; sparse staining in stromal macrophages. QuPath-0.5.0. DAB chromogen. Magnification ×100 |
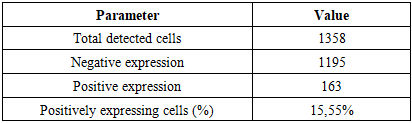 These findings indicate that monocytes and macrophages bearing the CD4 antigen—cells participating in T-lymphocytic immunity—are located in the lamina propria of the ileal mucosa and in the submucosa.In neonates who died on days 20–22 of NEC, the appearance of T lymphocytes in the lamina propria of the ileal mucosa was confirmed by low positive CD3 expression. These cells accumulated around postcapillary venules in the lamina propria, forming T-cell areas. At the same time, CD4-positive monocytes and macrophages were sparsely and diffusely expressed in other regions of the lamina propria. Because all layers of the ileal wall are diffusely affected in NEC, sparse CD4-positive monocytic/macrophagal cells and CD3-positive T lymphocytes were observed in the interstitial tissue across all layers, with weak reactions in some sites.Overall, examination of CD3+ and CD4+ markers showed low positive expression in MALT during NEC; values for the 0–7-day and 8–28-day periods were close to each other. The paucity of CD3+CD4+ T helpers indicates immunodeficiency and a marked reduction in resistance to infection. Based on these data, the process continues throughout days 0–28; in our study, nosocomial infection and the degree of intestinal injury were highest during days 8–28 of NEC.Ki-67 marker. Ki-67 is expressed in the perinuclear region of many cell types and serves as a marker of proliferative activity, playing an important role in evaluating the proliferation index of T lymphocytes. In immunohistochemistry, Ki-67 is expressed to varying degrees (light, moderate, strong brown) throughout all active phases of the cell cycle (G1, S, G2, M), with highest expression from early G1 to M and clear visualization at metaphase. In early G1, Ki-67 localizes to centromeric satellite DNA and telomeres; in mid-cycle phases it is detected intranuclearly within the nucleolus; by G2 it is expressed in the nucleolus and karyoplasm. After mitosis, as the cell enters G0, Ki-67 undergoes proteasomal degradation and is not expressed during interphase. In our work, this is critical for marking the proliferative phase during the formation of lymphocytes in MALT.Using Ki-67, a proliferation index can be calculated. With the modern QuPath-0.5.0 platform, automated commands allow counting of positively expressing nuclei at 200× and 400× magnification with 98.5% accuracy, minimizing human bias; the percentage of positively expressing cells is then computed.Grading by positive fraction:<10% — low;10–20% — moderate;>20% — high expression.
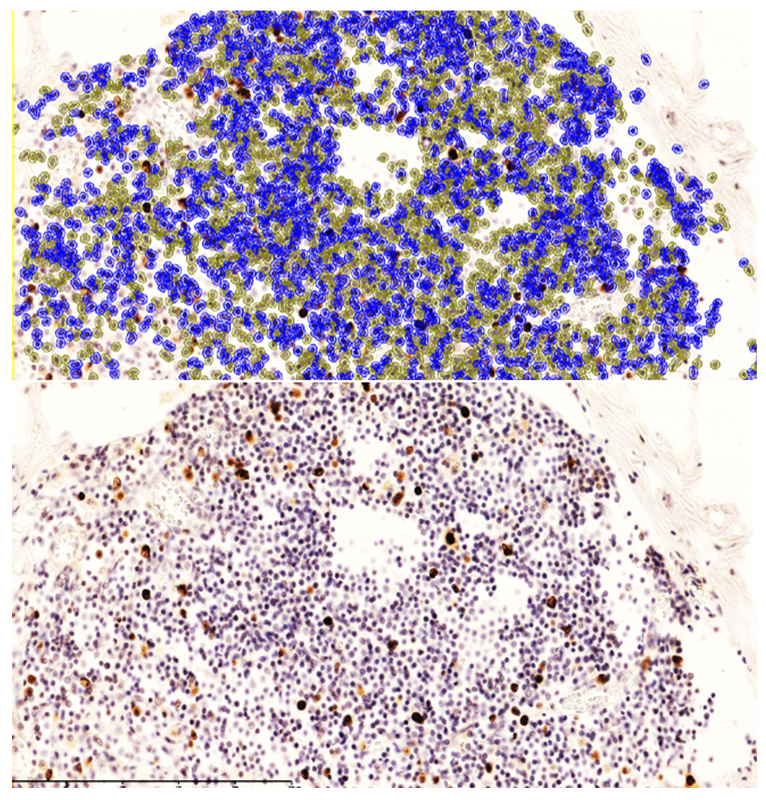
These findings indicate that monocytes and macrophages bearing the CD4 antigen—cells participating in T-lymphocytic immunity—are located in the lamina propria of the ileal mucosa and in the submucosa.In neonates who died on days 20–22 of NEC, the appearance of T lymphocytes in the lamina propria of the ileal mucosa was confirmed by low positive CD3 expression. These cells accumulated around postcapillary venules in the lamina propria, forming T-cell areas. At the same time, CD4-positive monocytes and macrophages were sparsely and diffusely expressed in other regions of the lamina propria. Because all layers of the ileal wall are diffusely affected in NEC, sparse CD4-positive monocytic/macrophagal cells and CD3-positive T lymphocytes were observed in the interstitial tissue across all layers, with weak reactions in some sites.Overall, examination of CD3+ and CD4+ markers showed low positive expression in MALT during NEC; values for the 0–7-day and 8–28-day periods were close to each other. The paucity of CD3+CD4+ T helpers indicates immunodeficiency and a marked reduction in resistance to infection. Based on these data, the process continues throughout days 0–28; in our study, nosocomial infection and the degree of intestinal injury were highest during days 8–28 of NEC.Ki-67 marker. Ki-67 is expressed in the perinuclear region of many cell types and serves as a marker of proliferative activity, playing an important role in evaluating the proliferation index of T lymphocytes. In immunohistochemistry, Ki-67 is expressed to varying degrees (light, moderate, strong brown) throughout all active phases of the cell cycle (G1, S, G2, M), with highest expression from early G1 to M and clear visualization at metaphase. In early G1, Ki-67 localizes to centromeric satellite DNA and telomeres; in mid-cycle phases it is detected intranuclearly within the nucleolus; by G2 it is expressed in the nucleolus and karyoplasm. After mitosis, as the cell enters G0, Ki-67 undergoes proteasomal degradation and is not expressed during interphase. In our work, this is critical for marking the proliferative phase during the formation of lymphocytes in MALT.Using Ki-67, a proliferation index can be calculated. With the modern QuPath-0.5.0 platform, automated commands allow counting of positively expressing nuclei at 200× and 400× magnification with 98.5% accuracy, minimizing human bias; the percentage of positively expressing cells is then computed.Grading by positive fraction:<10% — low;10–20% — moderate;>20% — high expression.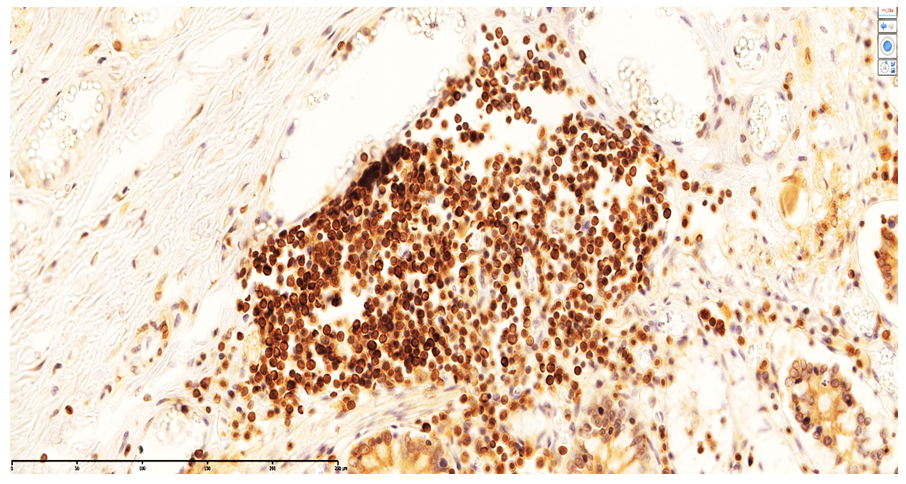 | Figure 4. Strong CD20 expression in B lymphocytes within MALT of a neonate who died on day 22 of NEC. DAB chromogen. 10×40 |
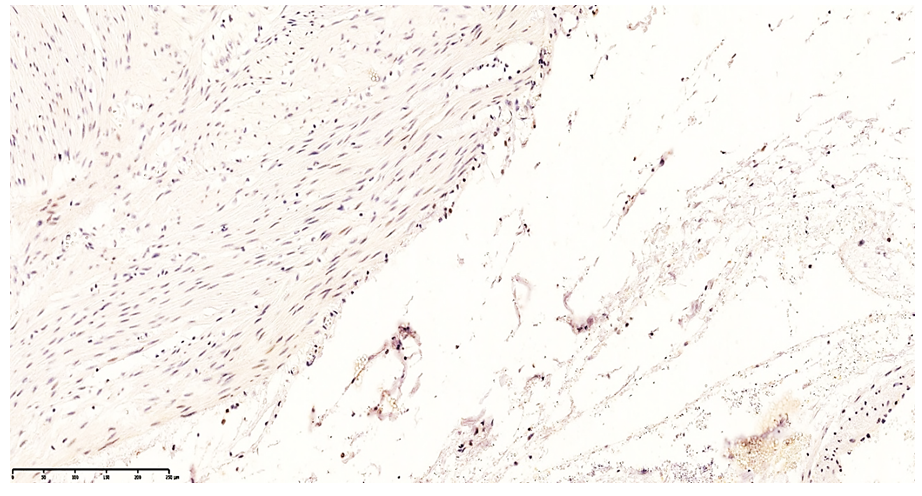 | Figure 5. Low-level CD20 expression in the lamina propria of the ileal mucosa. DAB chromogen. 10×40 |
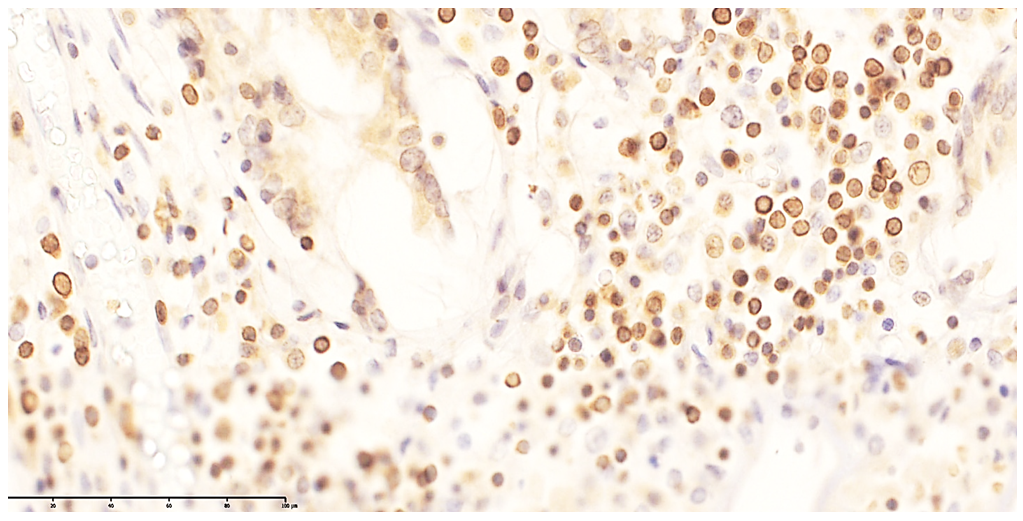 | Figure 6. Small-cluster CD20 expression in primary lymphoid follicles of the ileal mucosa. DAB chromogen. Magnification ×400 |
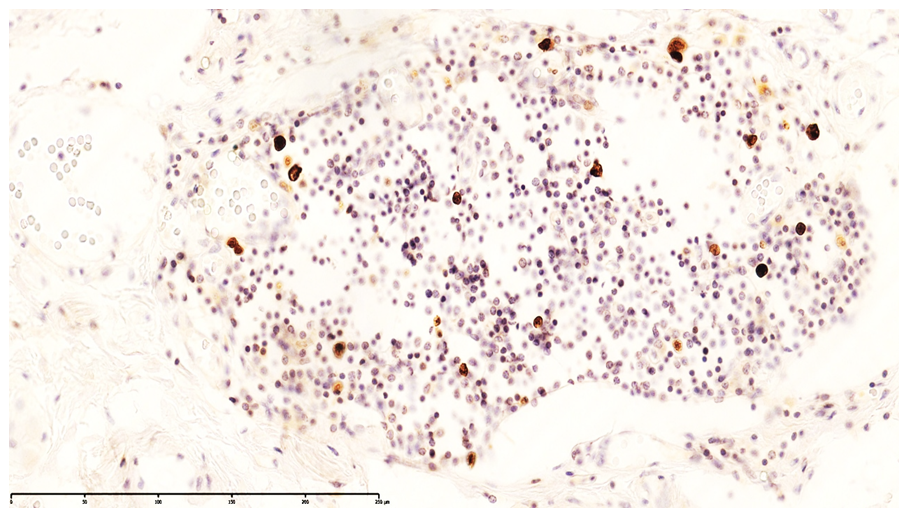
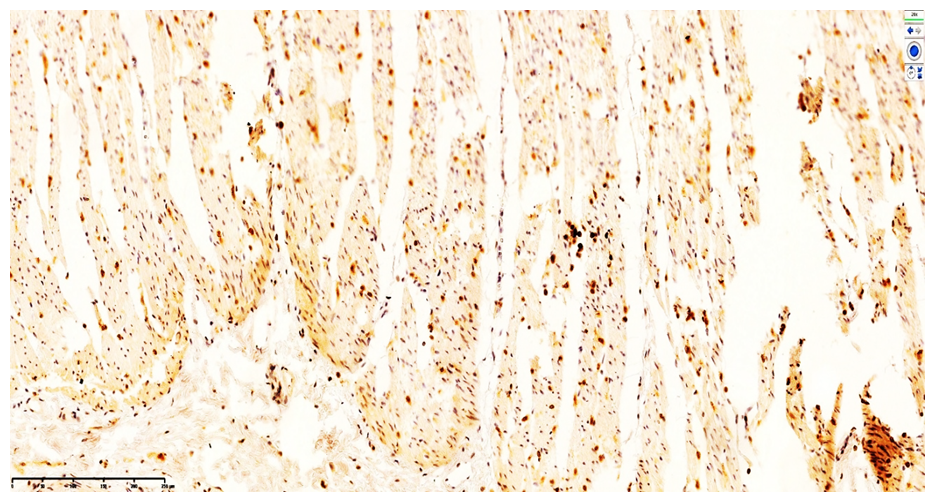 | Figure 7. CD4-positive monocytic/macrophagal and CD3-positive T-lymphocytic expression in the muscular layer of the ileum. DAB chromogen. Magnification ×400 |
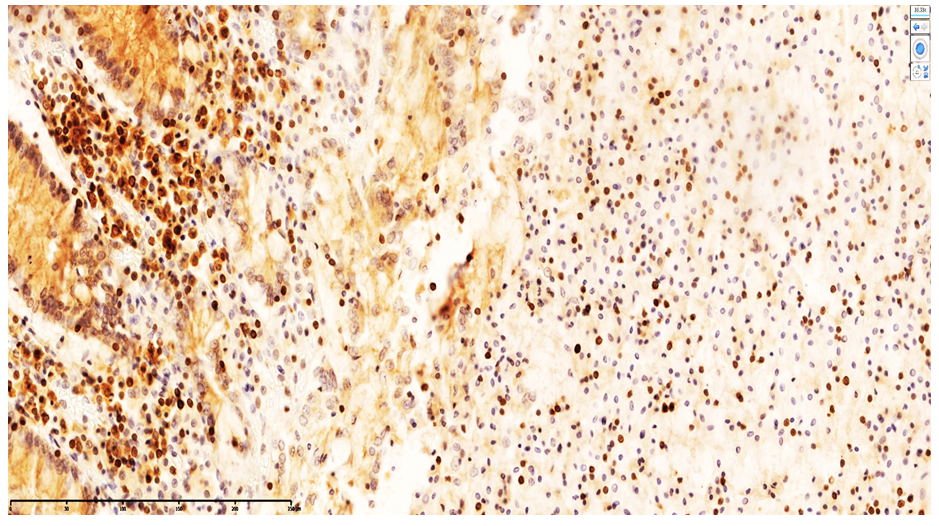 | Figure 8. Appearance of T lymphocytes in the lamina propria of the ileal mucosa confirmed by CD20-positive cell expression. DAB chromogen. Magnification ×400 |
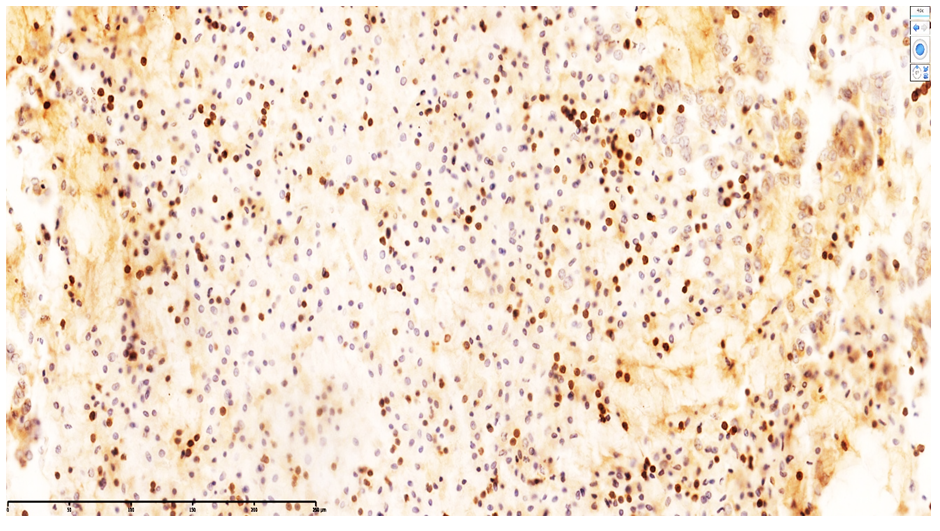 | Figure 9. Additional visualization of CD20-positive cells in the lamina propria of the ileal mucosa, indicating emerging T-cell areas. DAB chromogen. Magnification ×400 |
5. Conclusions
- Immunohistochemical studies show that, in NEC during the 0–7-day period, B lymphocytes are activated within primary lymphoid follicles of the ileal wall; initial intrafollicular CD20 expression indicates B-cell proliferation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML