-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(10): 3402-3406
doi:10.5923/j.ajmms.20251510.26
Received: Sep. 16, 2025; Accepted: Oct. 8, 2025; Published: Oct. 15, 2025

Clinical and Functional Characteristics of COPD Patients Depending on Renal Function
Muminov D. K., Daminova L. T., Tursunov D. I.
Tashkent State Medical University, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aim: To assess renal function and clinical–functional characteristics in COPD patients, focusing on early markers of kidney injury. Materials and Methods: We studied 130 COPD patients (≥40 years, stable phase), classified by GOLD (2019): stage I (n=48), stage II (n=52), and stage III (n=30). Twenty age-matched healthy individuals served as controls. Lung function was assessed by spirometry, MRC scale, CAT questionnaire, and six-minute walk test. Renal function was evaluated by serum creatinine, cystatin C, eGFR (CKD-EPI), and albumin-to-creatinine ratio (ACR). Doppler ultrasound measured renal parenchymal volume and resistance indices (RI). Patients were stratified into subgroups with preserved vs. impaired renal function. Results: At early COPD stages, creatinine and creatinine-based eGFR were within normal limits, while cystatin C revealed subclinical impairment. Compared with controls, cystatin C was significantly elevated in stages I–II (p<0.05), with reduced eGFRcys. Severe COPD patients showed marked decline: eGFRcr 78.3 vs. 97.0 mL/min in controls (p<0.01); eGFRcys ~68.4 mL/min (p<0.01). Overall, renal dysfunction was detected in 34.6% by creatinine vs. 49.2% by cystatin C. Microalbuminuria occurred in 12.3% and increased with COPD severity. Albuminuria and RI correlated with lower FEV₁, higher CAT and MRC scores, and reduced exercise tolerance (p<0.05). Conclusion: Renal impairment is common in COPD, even at early stages. Cystatin C and microalbuminuria are more sensitive than creatinine and strongly correlate with disease severity. Early renal screening may guide therapy and improve prognosis.
Keywords: COPD, Chronic kidney disease, Cystatin C, Microalbuminuria, Renal hemodynamics
Cite this paper: Muminov D. K., Daminova L. T., Tursunov D. I., Clinical and Functional Characteristics of COPD Patients Depending on Renal Function, American Journal of Medicine and Medical Sciences, Vol. 15 No. 10, 2025, pp. 3402-3406. doi: 10.5923/j.ajmms.20251510.26.
1. Introduction
- Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disorder characterized by systemic disturbances and comorbidities. In recent decades, the prevalence of chronic kidney disease (CKD) has been increasing, largely due to the rising incidence of chronic non-communicable diseases, including COPD. Studies have shown that the long course of COPD and the associated processes of systemic inflammation and hypoxia lead to kidney damage, thereby influencing the course and prognosis of the primary disease. According to a large population-based study, patients with COPD have approximately a 1.6-fold higher risk of developing CKD compared with individuals without COPD [1]. Renal impairment in COPD often remains undiagnosed due to the absence of overt symptoms and the lack of information regarding premorbid creatinine levels. Under these circumstances, the search for early markers of renal dysfunction in COPD patients becomes particularly relevant. One of the most promising biomarkers of early renal function decline is serum cystatin C. Unlike creatinine, cystatin C levels are independent of muscle mass, sex, age, diet, and other factors, and provide a more accurate reflection of the glomerular filtration rate (GFR). It has been shown that cystatin C concentrations are elevated in COPD and COPD exacerbations and inversely correlate with lung function parameters [2]. Another early indicator of kidney damage is microalbuminuria, i.e., the presence of small amounts of albumin in urine, which reflects endothelial dysfunction and glomerular injury. In COPD patients, microalbuminuria occurs more frequently, and its levels rise with increasing disease severity [3]. Moreover, microalbuminuria is regarded as a marker of elevated cardiovascular risk and poor prognosis in COPD. Finally, chronic systemic inflammation in COPD may contribute to atherosclerotic vascular damage, including lesions of the renal arteries, leading to increased vascular resistance and reduced renal perfusion [1]. A non-invasive method for assessing intrarenal hemodynamics is Doppler ultrasonography with determination of the resistance index (RI) in the renal arteries. An elevated RI correlates with renal vascular injury and adverse prognosis in kidney disease. Thus, renal dysfunction is a frequent and underestimated component of the systemic manifestations of COPD. Early detection of declining renal function in this patient population is of great importance for optimizing treatment strategies and improving prognosis [1]. Early detection of renal impairment in COPD patients allows timely adjustment of therapy, avoidance of nephrotoxic exposure, and improvement of long-term prognosis.The aim of this study was to assess renal function and clinical–functional characteristics in COPD patients, focusing on early markers of kidney injury. The present study is devoted to the investigation of the clinical and functional characteristics of COPD patients depending on renal function, with special emphasis on early markers of kidney injury (cystatin C, microalbuminuria) and their association with COPD severity. Aim of the study to assess renal function and the clinical–functional characteristics of COPD patients depending on the stage of kidney function.
2. Materials and Methods
- The study included 130 patients with COPD (men and women over 40 years of age) in the stable phase of the disease, who were observed in a specialized hospital (tertiary care center providing advanced diagnostic and treatment services). The diagnosis of COPD was established according to GOLD (2019) criteria using spirometry. Depending on the severity of bronchial obstruction, patients were divided into groups: COPD stage I (mild, n=48), stage II (moderate, n=52), and stage III (severe, n=30), based on the percentage of predicted FEV₁. The control group consisted of 20 practically healthy individuals of similar age without evidence of lung or kidney disease. All participants underwent clinical and instrumental examination. Lung function was assessed using spirometry by measuring forced vital capacity (FVC) and forced expiratory volume in one second (FEV₁), and calculating the Tiffeneau index (FEV₁/FVC, %). COPD symptom severity was assessed with the MRC dyspnea scale and the CAT questionnaire, while exercise tolerance was evaluated by the six-minute walk test.To assess kidney function, fasting serum creatinine and cystatin C levels were measured in all patients. Estimated glomerular filtration rate (eGFR) was calculated by two methods: creatinine-based (eGFRcr, mL/min) and cystatin C–based (eGFRcys, mL/min), using standard CKD-EPI equations. A reduced filtration function was defined as eGFR <90 mL/min (any degree of nephropathy according to the KDIGO CKD classification). Urinary albumin was measured in a single morning urine sample, expressed as the albumin-to-creatinine ratio (ACR, mg/g). Microalbuminuria was defined as urinary albumin of 30–300 mg/g. Ultrasound examination of the kidneys with Doppler ultrasonography of renal arteries was performed. The renal parenchymal volume index (RPVI, cm³/m²) and resistance indices of the main renal artery (RI-MRA) and segmental arteries (RI-SA) were measured in both kidneys. A normal RI was defined as <0.70.For the analysis of clinical–functional characteristics according to renal function, COPD patients were divided into two subgroups: those with preserved renal function and those with renal dysfunction. The latter included patients with evidence of CKD (eGFR <90 mL/min and/or pathological albuminuria). Pulmonary function, test results, and scale scores were compared between these groups. Statistical analysis included testing for normal distribution, application of Student’s t-test (for normally distributed variables) or the Mann–Whitney U-test (for non-normally distributed variables) for quantitative parameters, and the chi-square test for categorical variables. Correlation analysis was performed using Pearson’s correlation coefficient (r). Differences were considered statistically significant at p<0.05.
3. Results
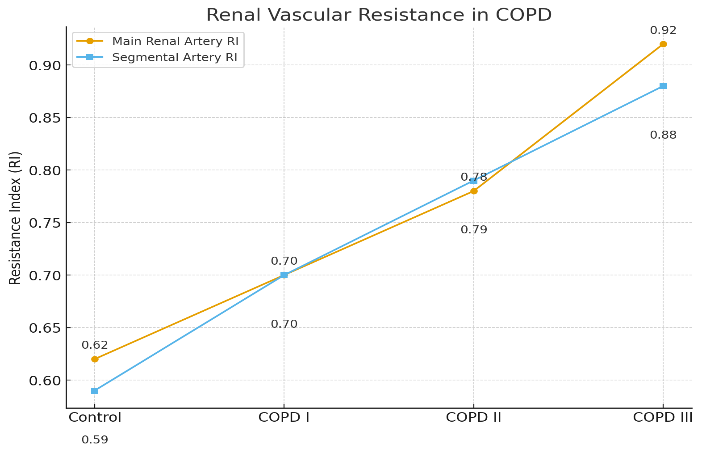
- In most patients with mild and moderate COPD, serum creatinine levels remained within the normal range, and creatinine-based eGFR did not differ from controls, indicating preserved renal function at these stages. However, the more sensitive marker—serum cystatin C—revealed early signs of reduced filtration already at the initial stages of the disease. Patients with COPD stage I–II demonstrated a significant increase in cystatin C compared with controls (p<0.05), accompanied by a decrease in eGFRcys. Importantly, differences in cystatin C and eGFRcys were significant not only relative to controls but also between the mild and moderate COPD groups (p<0.05). This indicates that even at the early stages of COPD, about one-third of patients show hidden reductions in renal filtration. Cystatin C levels increased proportionally with COPD severity, showing a progressive rise from mild to severe disease.As COPD progressed, renal dysfunction became more pronounced. In the severe COPD group (stage III), serum creatinine was elevated, and eGFRcr was significantly reduced. For example, in severe COPD, mean eGFRcr was significantly lower than in controls (78.3 vs. 97.0 mL/min, p<0.01) and lower than in stage I patients (p<0.05). Even more marked changes were seen with cystatin C: in severe COPD, mean eGFRcys was ~68.4 mL/min, significantly lower than controls and both stage I–II groups (p<0.01). Thus, reductions in eGFRcys were detected earlier and more frequently than creatinine-based estimates, confirming the advantage of cystatin C as an early indicator of renal impairment.The proportion of patients with reduced renal filtration increased with COPD severity. Across all COPD patients, reduced eGFRcr (<90 mL/min) was found in 34.6%, while reduced eGFRcys was seen in 49.2%. Even among those with mild COPD, 14.6% already showed reduced eGFRcr, indicating early CKD. According to KDIGO CKD classification, 66.9% of COPD patients had eGFRcr >90 mL/min with normoalbuminuria (CKD stage C1A1), 22.3% had moderately decreased eGFR 60–89 mL/min with normoalbuminuria (C2A1), and 12.3% had both decreased eGFR (60–89 mL/min) and microalbuminuria >30 mg/g (C2A2). Thus, one-third of COPD patients already exhibited signs of CKD, mostly early stages (stage 2 CKD with microalbuminuria). With disease progression, a mixed-genesis CKD developed, combining COPD-related factors with traditional renal risk factors.Urinary albumin and the albumin-to-creatinine ratio (ACR) in most COPD patients did not exceed threshold values for microalbuminuria (<30 mg/g albumin, <3 mg/mmol ACR). However, even within the normal range, there was a clear trend toward increasing values with COPD severity. In moderate COPD, mean albuminuria was higher than in mild COPD and significantly different from controls (p<0.01). In severe COPD, mean urinary albumin rose further (~22.6±3.3 mg/g), nearly five times higher than controls (p<0.001). Similarly, mean ACR increased from 0.9±0.08 mg/mmol in mild COPD to 2.5±0.06 mg/mmol in severe COPD, remaining below the microalbuminuria threshold but significantly higher than controls (p<0.01–0.001). These findings confirm that glomerular injury with increased albumin permeability occurs with COPD progression. In some patients, particularly with severe COPD, true microalbuminuria (>30 mg/g) was observed, reflecting more advanced kidney injury. Serum urea levels in COPD stage I–II did not differ from controls, but in severe COPD, urea was moderately elevated (8.64±1.1 vs. 5.23±1.06 mmol/L, p<0.05). Serum uric acid levels tended to rise in moderate COPD and were significantly elevated in severe COPD (~410±20 µmol/L vs. 237±18 µmol/L in controls, p<0.001, and vs. stage I, p<0.01). Hyperuricemia in severe COPD reflects impaired tubular uric acid excretion with worsening renal function. Moreover, chronically elevated uric acid itself may contribute to CKD progression as an independent risk factor. These data complement the observed decline in renal excretory function in severe COPD. Hyperuricemia in COPD patients results from impaired renal tubular excretion, systemic hypoxia, and increased oxidative stress.Ultrasound examination of kidney size and parenchyma showed no significant abnormalities; the renal parenchymal volume index (RPVI) was within normal limits and did not differ across groups. However, Doppler indices of renal blood flow changed markedly with severe COPD.
 | Figure 1 |
4. Discussion
- The present findings demonstrate that chronic renal dysfunction is highly prevalent among COPD patients and tends to worsen with disease progression. Even at early stages of COPD, a considerable proportion of patients show subclinical signs of renal impairment—primarily detected through sensitive biomarkers such as cystatin C and albuminuria. For instance, in mild and moderate COPD, serum creatinine levels may remain within normal limits; however, elevated cystatin C and the onset of microalbuminuria already indicate early renal injury [7]. This is consistent with the literature: reductions in eGFR among COPD patients often remain unrecognized when based on creatinine due to reduced muscle mass in this population, whereas cystatin C provides a more accurate assessment of early CKD. In our study, the prevalence of renal dysfunction was nearly 1.5 times higher when using cystatin C compared to creatinine-based estimates (49% vs. 35%). Thus, cystatin C should be considered a more reliable screening test for renal function in COPD patients, especially in elderly and sarcopenic individuals, where creatinine may underestimate the degree of impairment. The presence of even moderately reduced renal function has an adverse impact on COPD. We found that COPD patients with CKD had worse lung function (lower FVC and FEV₁), more severe dyspnea, and reduced exercise tolerance compared with those without renal pathology. Similar results have been reported by others; for example, decreased eGFR in smokers has been linked to more severe emphysematous changes. The mutual aggravation of pulmonary and renal dysfunction may be explained by several pathophysiological mechanisms. Chronic hypoxemia in advanced COPD contributes to renal vascular injury, activation of the renin–angiotensin system, and increased sodium reabsorption, thereby promoting CKD progression [8]. Conversely, impaired renal function aggravates systemic inflammation and oxidative stress and may lead to anemia and electrolyte disturbances, all of which negatively affect respiratory function. Our findings of significant correlations between declining renal parameters (eGFR, albuminuria, RI) and greater dyspnea, lower exercise tolerance, and reduced FEV₁ confirm this bidirectional relationship. Conversely, impaired renal function aggravates systemic inflammation, oxidative stress, anemia, and electrolyte imbalance, further worsening respiratory symptoms and lung function in COPD.Of particular importance is the observed increase in renal artery resistance indices in COPD patients, especially in severe disease. Elevated RI reflects increased resistance in renal arterioles, which may result from generalized atherosclerosis and microangiopathy [1]. COPD is known as a systemic disorder associated with accelerated atherosclerosis driven by chronic inflammation. Previous studies have also shown that even in smokers with moderate COPD, the so-called renal functional reserve is impaired: following protein load, they fail to reduce renal vascular resistance adequately, and instead exhibit prolonged RI elevation, particularly in those with lower FEV₁ and higher TNF-α levels [4]. Our results are in agreement, showing maximal RI values in patients with the most severe bronchial obstruction. Increased RI correlated with lower six-minute walk distances and reduced FEV₁, indirectly linking systemic vascular involvement to pulmonary dysfunction. Thus, Doppler assessment of renal hemodynamics may serve as an additional tool for risk stratification in COPD: elevated RI may reflect systemic vascular burden and partially predict adverse outcomes (since elevated renal RI is associated with cardiovascular mortality in hypertension and CKD) [5].It should be emphasized that the identified abnormalities—reduced eGFR, microalbuminuria, and increased RI—were already present at early COPD stages, when clinical focus is usually directed mainly at respiratory symptoms. In our data, even patients with mild and moderate obstruction had significantly higher cystatin C and urinary albumin-to-creatinine ratios compared to controls. This underscores the need for early renal screening in all COPD patients, regardless of respiratory symptom severity. Early detection of renal impairment would allow timely therapeutic adjustments, such as avoidance of nephrotoxic drugs, stricter blood pressure control, and correction of hyperuricemia and other factors, potentially slowing CKD progression. Moreover, the presence of CKD influences COPD treatment strategies (dose adjustments for some drugs are required in reduced eGFR) and prognosis. According to meta-analyses, comorbid CKD increases the risk of hospitalization and mortality in COPD [6]. Therefore, an integrated approach that accounts for renal status is essential in the management of COPD patients. According to meta-analysis [6], cystatin C levels are strongly associated with COPD and represent a reliable biomarker of early renal impairment, supporting our findings.
5. Conclusions
- Renal dysfunction is frequently observed in COPD patients and worsens with disease progression. Even at early stages, reductions in eGFR and the presence of microalbuminuria can be detected, with cystatin C being more sensitive than creatinine. Increased albuminuria and decreased eGFR are associated with worse lung function and more severe clinical manifestations, confirming the interplay between pulmonary and renal injury. Early detection and correction of renal dysfunction cystatin C and microalbuminuria testing may improve outcomes in COPD patients. Therefore, routine early screening with cystatin C is recommended in COPD patients to detect renal dysfunction at initial stages.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML