-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(10): 3372-3376
doi:10.5923/j.ajmms.20251510.19
Received: Aug. 25, 2025; Accepted: Sep. 16, 2025; Published: Oct. 15, 2025

Clinical and Genetic Characteristics of Patients with Gout and Kidney and Liver Damage
Shodikulova Gulandom Zikriyayevna, Pulatov Ulugbek Sunatovich, Karimov Aziz Khamrokulovich
Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Shodikulova Gulandom Zikriyayevna, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

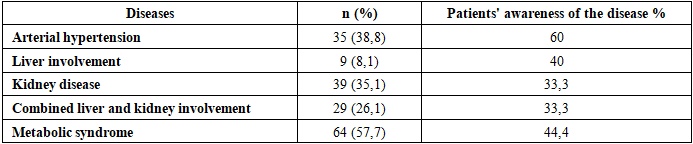
Introduction. Gout is a condition that involves inflammation in the joints and the build-up of uric acid crystals in different tissues, which causes serious pain, swelling, and inflammation. Gout is one of the oldest conditions known to humans. Among rheumatic diseases, gout is considered the most studied, understood, and well-controlled nosologically entity. Research materials and methods. The research work was carried out at the central hospital of the Samarkand City Medical Association. For the dissertation work, a comprehensive approach was used, including clinical, laboratory, biochemical, immunological, ultrasound, radiological, CT, and statistical research methods. The study examined 111 patients with primary gout, who were divided into 3 groups: 1st group (n=34), patients diagnosed with primary gout without damage to internal organs. 2nd group (n=39), patients with primary gout with kidney damage and patients with primary gout with liver and kidney damage 3rd group (n=38). Research results. The average age of the patients was 55.9±8 years (from 29 to 65 years). Patients aged up to 43 years - 21.33%, from 44 to 58 years - 52%, from 59 to 65 years - 26.67%. The average age of patients at the onset of the disease was 44.8±8 years. In most patients (71.1%) the onset of the disease was observed on average at the age of 35-52 years. The average duration of the disease upon admission was 5.2 (1.0;10.0 years). Conclusions. Thus, our results showed no association between the Ala22Val polymorphism in the MTHFR gene and the development of gout in our patients, which contradicts the data of some scientific studies, where a predisposing effect of the mutant Val allele on the development of GU and gout in the adult population was found.
Keywords: Gout, Liver pathology, Chronic kidney disease, Clinical course, Genetic predisposition
Cite this paper: Shodikulova Gulandom Zikriyayevna, Pulatov Ulugbek Sunatovich, Karimov Aziz Khamrokulovich, Clinical and Genetic Characteristics of Patients with Gout and Kidney and Liver Damage, American Journal of Medicine and Medical Sciences, Vol. 15 No. 10, 2025, pp. 3372-3376. doi: 10.5923/j.ajmms.20251510.19.
1. Introduction
- Gout is a condition that involves inflammation in the joints and the build-up of uric acid crystals in different tissues, which causes serious pain, swelling, and inflammation. Gout is one of the oldest conditions known to humans. Among rheumatic diseases, gout is considered the most studied, understood, and well-controlled nosologically entity [19]. The epidemiology of gout varies depending on the region and the population studied. According to Dehlin, M et al. (2020), various factors influence the prevalence and incidence of gout (location of the study group, genetics, research methodology, etc.), but it is known that the indicators vary in the range of <1% to 6.8% and 0.58-2.89 per 1,000 person-years, respectively. Gout is more common in men than in women, with increasing age and in some ethnic groups [20]. In recent decades, there has been an active search for candidate genes associated with the development of hyperuricaemia (GU) and gout, and the influence of genetic factors on the regulation of uric acid (UA) synthesis and excretion has been studied [2]. Polymorphic loci encoding folate metabolism can be considered as candidate genes predisposing to the development of HU and gout [4,8]. Folate metabolism disorders are associated with an increased risk of cardiovascular diseases (coronary heart disease, atherosclerosis, stroke), haemostasis system pathologies, pregnancy complications, osteoporosis, and rheumatoid arthritis [11,14]. On the other hand, the participation of folates in the biosynthesis of purine nucleotides suggests a possible role for the folate cycle in the pathogenesis of GU and gout.
2. Research Materials and Methods
- The research work was carried out at the central hospital of the Samarkand City Medical Association. For the dissertation work, a comprehensive approach was used, including clinical, laboratory, biochemical, immunological, ultrasound, radiological, CT (computer tomography), and statistical research methods. The study examined 111 patients with primary gout, who were divided into 3 groups: 1st group (n=34), patients diagnosed with primary gout without damage to internal organs. 2nd group (n=39), patients with primary gout with kidney damage and patients with primary gout with liver and kidney damage 3rd group (n=38). Biochemical research methods (renal and hepatic parameters, lipid profile, rheumatic trials, uric acid level, IL6, IL10, TNF-α levels).Assessment of the functional state of the joints was carried out according to a special questionnaire; the functional index of the foot, the American Orthopedic Foot and Ankle Joint Society (AOFAS) scale [9,12]. The AOFAS scale assesses parameters: pain, functional limitations, mobility, and some aspects of quality of life [11,13,14]. Assessment of the quality of life was carried out using the European Quality of Life Questionnaire (EQ-5D), since this questionnaire is universal and allows for a real assessment of the patient's psychometric state (reliability, validity, sensitivity) [6-8]. The Gout Impact Scale (GIS) was also used - a method specific to gout for assessing not only the quality of life, but also the impact of gout at the time of the attack and in general. The questionnaire was included in the form of five scales.Statistical processing of the results was carried out on a personal computer using the "Statistica 6.0" software package with the calculation of the arithmetic mean (M), the error of the arithmetic mean (m), Student's t-test (t), and the equality of total variances (F - Fisher's criterion). A significance level of R=0.05 was taken as statistically significant changes. For statistical analysis of the obtained research results, statistical packages Statistica 12.0, Microsoft Excel 2010 were used.
3. Research Results
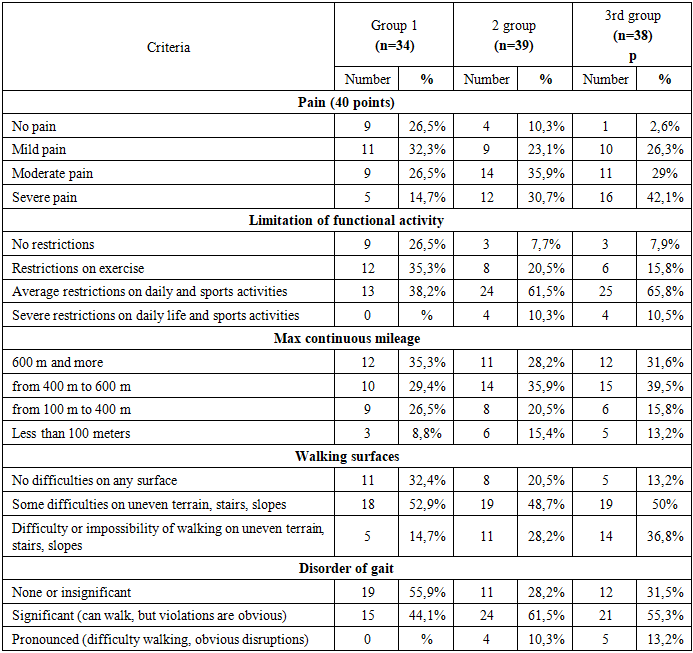
- The average age of the patients was 55.9±8 years (from 29 to 65 years). Patients aged up to 43 years - 21.33%, from 44 to 58 years - 52%, from 59 to 65 years - 26.67%. The average age of patients at the onset of the disease was 44.8±8 years. In most patients (71.1%) the onset of the disease was observed on average at the age of 35-52 years. The average duration of the disease upon admission was 5.2 (1.0;10.0 years). The general characteristics of patients by age and duration of the disease are presented in Table 1.
|
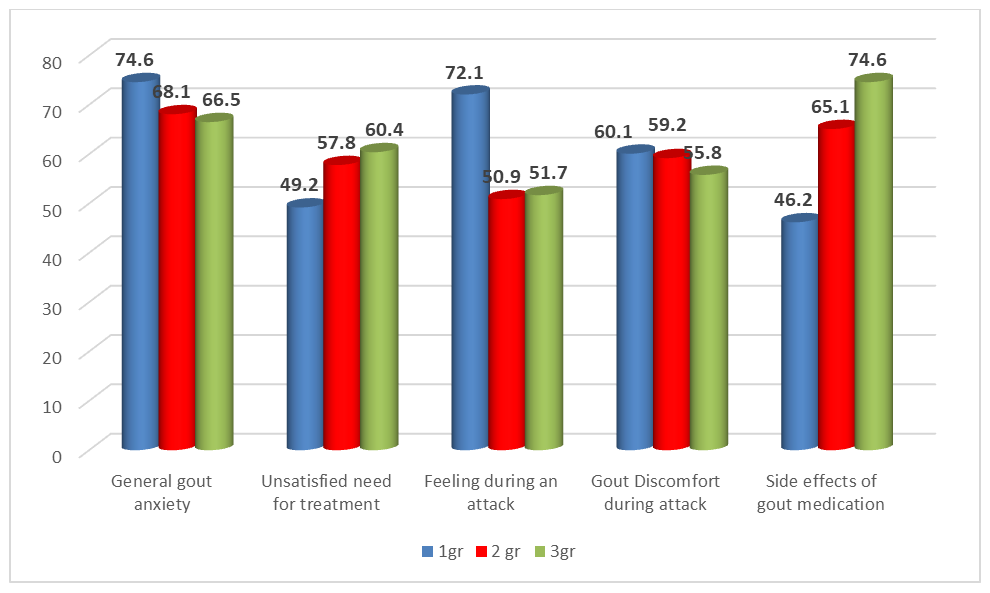 | Figure 1. Gout Impact Scale (GIS) indicators |
|
|
4. Conclusions
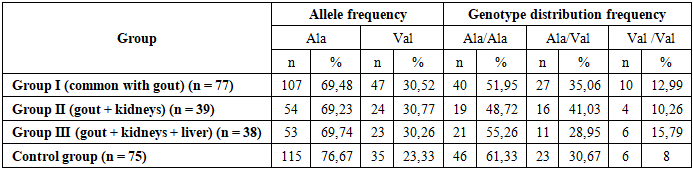
- Thus, our results showed no association between the Ala22Val polymorphism in the MTHFR gene and the development of gout in our patients, which contradicts the data of some scientific studies, where a predisposing effect of the mutant Val allele on the development of GU and gout in the adult population was found. In our scientific work, despite the prevalence of the mutant Val allele and the Val/Val genotype of the MTHFR gene in the group of patients with gout, we did not find any significant genetic associations with gout. Information about the source of support in the form of grants, equipment, and drugs. The authors did not receive financial support from manufacturers of medicines and medical equipment.Conflicts of interest. The authors have no conflicts of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML