-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(9): 3047-3051
doi:10.5923/j.ajmms.20251509.43
Received: Aug. 19, 2025; Accepted: Sep. 15, 2025; Published: Sep. 25, 2025

Study of Morphological Changes in the Lungs of White Outbred Rats Under the Influence of Timolin After Standard Chemotherapy
Shomurodova Muhayyo Rahmonovna
Assistant, Department of Pathological Anatomy, Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Shomurodova Muhayyo Rahmonovna, Assistant, Department of Pathological Anatomy, Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study investigated morphological changes in the lung tissues of white outbred rats after standard chemotherapy, as well as the possibilities of their correction using the drug Timolin. During the experiment, following the administration of chemotherapeutic agents, degenerative, inflammatory, and dystrophic processes were observed in the alveolar structures, interstitial tissue, and blood vessels. In the group treated with Timolin, morphological recovery of the lung parenchyma, normalization of alveolar size, stromal components, and positive dynamics of morphometric parameters were noted. The results confirm the cytoprotective and immunomodulatory properties of Timolin and demonstrate its potential effectiveness in alleviating post-chemotherapy complications.
Keywords: Chemotherapy, Timolin, Lungs, Morphology, Morphometry, Immunomodulator
Cite this paper: Shomurodova Muhayyo Rahmonovna, Study of Morphological Changes in the Lungs of White Outbred Rats Under the Influence of Timolin After Standard Chemotherapy, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 3047-3051. doi: 10.5923/j.ajmms.20251509.43.
Article Outline
1. Introduction
- In the 21st century, cancer is considered one of the most pressing problems in the global healthcare system. In recent years, the incidence of oncological diseases has been rapidly increasing, and this situation has become an urgent issue not only in developed but also in developing countries. Chemotherapy, as one of the main methods of cancer treatment, stands out for its high effectiveness; however, it also causes numerous adverse effects. Chemotherapeutic agents exert toxic effects not only on tumor cells but also on normal tissues characterized by rapid cell division and regenerative capacity. Therefore, post-chemotherapy complications are regarded as one of the major clinical and theoretical problems of modern oncology [1,2,3,4,5,6].During chemotherapy, various organs of the body, including lung tissue, undergo morphofunctional changes. In the lung parenchyma, disruption of alveolar structures, interstitial inflammation, and dystrophic and degenerative processes in stromal tissues significantly impair respiratory function. This negatively affects patients’ quality of life, making it difficult to continue chemotherapy courses or forcing their interruption. Thus, preventing or correcting chemotherapy-induced lung damage represents a scientifically and practically relevant issue [7,8,9].In recent years, increasing attention has been drawn to the role of immunomodulatory drugs in alleviating the consequences of chemotherapy. Among them, thymus-derived peptide preparations—particularly thymalin—stand out for their cytoprotective properties, regulating immune responses and protecting tissues. Thymalin supports the activity of immunocompetent cells, enhances phagocytic activity, activates antioxidant and reparative processes, thereby strengthening not only general immunity but also the regenerative capacity of tissues. From this perspective, studying the morphological and morphometric changes in the lungs after chemotherapy and their correction using thymalin is considered a highly relevant scientific direction [10,11].The significance of this study lies in its ability to provide an in-depth experimental investigation of morphofunctional processes occurring in lung tissue after chemotherapy, to evaluate them using morphological and morphometric parameters, and to determine the immunomodulatory and cytoprotective effects of thymalin. The obtained results will have substantial theoretical and practical importance in developing new approaches aimed at reducing the adverse consequences of chemotherapy, as well as in supporting respiratory function in cancer patients in clinical practice [12,13,14].
2. Purpose of the Study
- The aim of this study is to identify the morphological changes occurring in the lung tissue of white outbred rats after conventional (standard) chemotherapy and to evaluate the possibilities of correcting these changes with the use of the thymalin preparation.
3. Materials and Methods
- Within the framework of the experiment, 30 female white outbred rats aged 4 to 7 months and weighing up to 200 grams were used. During the study, the strict standards of animal care, handling, and grouping established in the works of Nuraliev N.A. and his colleagues, in accordance with biosafety rules and ethical principles, were followed. To exclude possible diseases, all laboratory animals underwent veterinary examination. To prevent external infections, the rats were kept under quarantine for 21 days.As part of the research project, the laboratory rats were divided into 3 groups:1. Control group (n=10): healthy white outbred rats, taken for comparison with the experimental groups.2. Main experimental group 1 (n=10): breast cancer was induced in rats using the carcinogenic agent 7,12-dimethylbenz[a]anthracene. The presence of the disease was verified by determining the CA-15-3 (Cancer Antigen 15-3) tumor marker in the blood.
 | Picture 1. Modeling of breast cancer in experimental rats using the carcinogenic agent 7,12-dimethylbenz[a]anthracene |
4. Results and Discussion
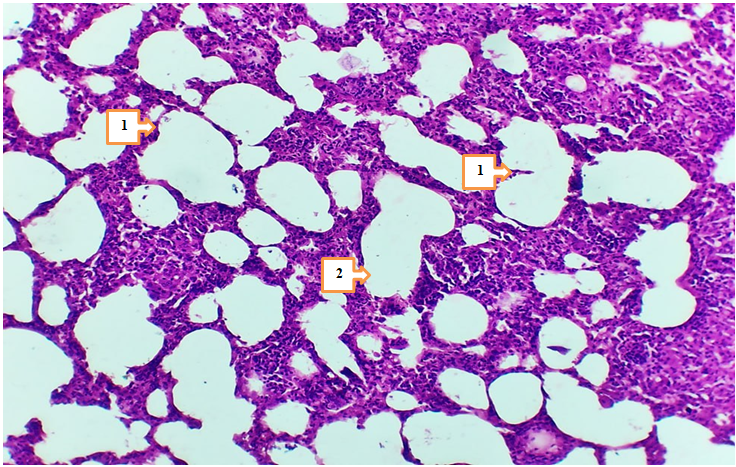
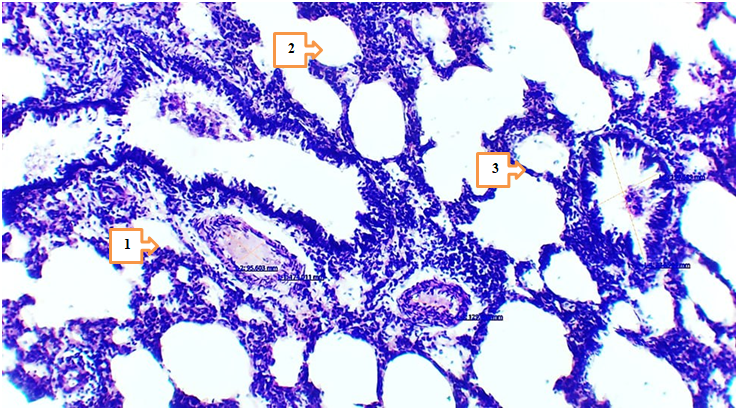
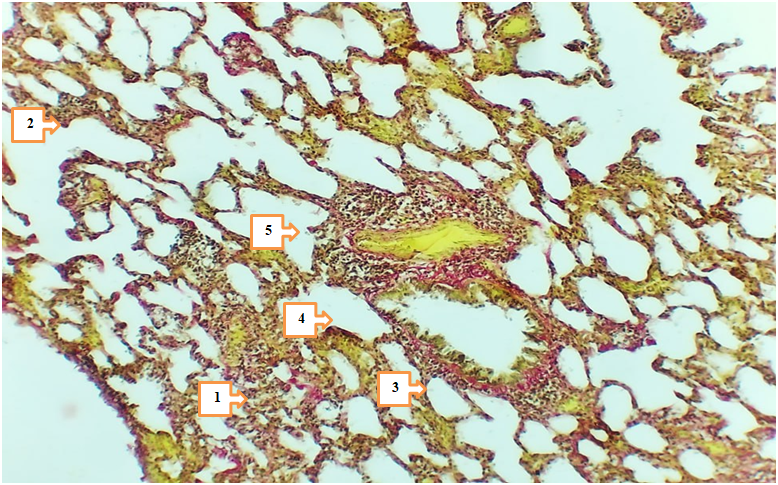
- In 4- and 7-month-old rats treated with Cisplatin in combination with Thymalin, signs of tissue stability were observed in certain aspects of metabolic and inflammatory changes in lung tissue. The height of mesothelium was measured at 2.97–3.36 nm in 4-month-old rats and 3.15–3.47 nm in 7-month-old rats, with less pronounced vacuolization and nuclear hyperchromasia in mesothelial cells. Degeneration of epithelial cells was noted only in isolated cases, which may be associated with the partial improvement of trophic functions under the influence of Thymalin.
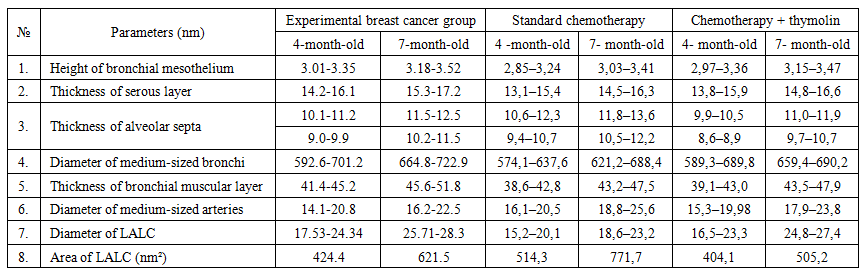 | Table 1 |
5. Conclusions
- The results of the conducted study demonstrated that standard chemotherapy (cisplatin) combined with thymolin significantly alleviates the morphological consequences of breast cancer in the lungs of white outbred rats. Particularly, at 4- and 7-month observations, active regenerative processes in lung tissue and a reduction of inflammatory signs were observed.Correction of breast cancer with standard chemotherapy (cisplatin) in combination with thymolin led to active regeneration and attenuation of inflammation in the lung tissue of 4- and 7-month-old white outbred rats. Based on morphometric indicators, the following changes were recorded: the height of the mesothelium was 2.97–3.36 nm and 3.15–3.47 nm, respectively; the thickness of the serous layer was 13.8–15.9 nm and 14.8–16.6 nm. The thickness of the alveolar septa and the degree of infiltration decreased. The bronchial diameter measured 589.3–689.8 nm (at 4 months) and 659.4–690.2 nm (at 7 months), while the muscular layer thickness was 39.1–43.0 nm and 43.5–47.9 nm, respectively. The arterial diameter was around 16.3–20.7 nm and 18.9–25.8 nm. Microhemorrhages in blood vessels decreased. The number of LALT remained unchanged (28–35%), and the area of lipid cells was 404.1–505.2 nm². These changes indicate the anti-inflammatory and trophic effects of thymolin.Endothelial damage in blood vessels and the number of diapedetic microhemorrhages were significantly reduced. In the terminal bronchioles, trophic disturbances of the epithelium were observed to a lesser degree, the amount of serous substance within the bronchi was reduced, and the degree of infiltration decreased.These findings confirm the trophic, immunomodulatory, and anti-inflammatory effects of thymolin. Under its influence, morphological and morphometric features of lung tissue remodeling and stabilization were clearly expressed.According to morphometric analysis, the mesothelial height and thickness of the serous layer approached normal ranges, the alveolar septa became thinner, and the degree of infiltration decreased, which indicates the positive effect of thymolin. Restoration of bronchial and arterial diameters, as well as normalization of muscular layer thickness, demonstrated improvement of ventilation and circulation in the respiratory pathways. Reduced endothelial damage and diapedetic microhemorrhages revealed the angioprotective and anti-inflammatory properties of thymolin.Moreover, a decrease in epithelial trophic disturbances in the terminal bronchioles, reduced accumulation of serous material inside the bronchi, and decreased infiltration indicated the activation of stabilization processes in lung tissue. The relative constancy of the number of LALT (28–35%) confirmed the preservation of the immunological defense potential of the lungs, while stabilization of the lipid cell area reflected balanced trophic processes.In general, complex therapy with thymolin not only reduced inflammatory reactions in lung tissue but also enhanced regenerative processes, decreased trophic disturbances, and contributed to the normalization of morphofunctional parameters. These results confirm the effective immunomodulatory, trophic, and anti-inflammatory action of thymolin and demonstrate its potential as a promising agent in alleviating the consequences of chemotherapy in breast cancer.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML