Tosheva Dilnoza Raxmatovna
Assistant, Department of Microbiology, Virology and Immunology of the Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Tosheva Dilnoza Raxmatovna, Assistant, Department of Microbiology, Virology and Immunology of the Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
All diagnoses of pulmonary tuberculosis were confirmed not only bacteriologically, but also through radiographic examination performed on all patients. Since these instrumental studies were not included in the objectives and tasks of our dissertation research, we considered it appropriate not to present this data. The final diagnoses were confirmed by the attending phthisiatricians based on the analysis of all clinical, radiological, and bacteriological indicators for each patient.
Keywords:
Mycobacterium tuberculosis, Strain, Lowenstein–Jensen, Ziehl–Neelsen, Rifampicin, Culture, Sputum
Cite this paper: Tosheva Dilnoza Raxmatovna, Materials and Research Methods Used in the Study of the Microbiological Aspects of Pulmonary Tuberculosis, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 3042-3046. doi: 10.5923/j.ajmms.20251509.42.
1. Introduction
To conduct this study, 315 patients diagnosed with primary and secondary tuberculosis who received inpatient treatment in the departments of phthisiotherapy, pulmonology, and surgery at the Bukhara Regional Center of Phthisiology and Pulmonology during 2022–2023 were enrolled [1,2,3,4,5].Among the examined individuals, 195 (42.86±4.08%) were male, and 120 (57.14±4.08%) were female. Additionally, 27 patients (18.37±3.19%) were permanent residents of urban areas, while 120 patients (81.63±3.19%) lived in rural areas [6,7].The ratio of males to females, as well as the distribution by place of residence, does not reflect the true epidemiological picture of tuberculosis, since our research focuses solely on the immunological and hematological aspects of the disease and does not aim to study the prevalence of tuberculosis in the region under investigation [8,9].
2. Material and Method
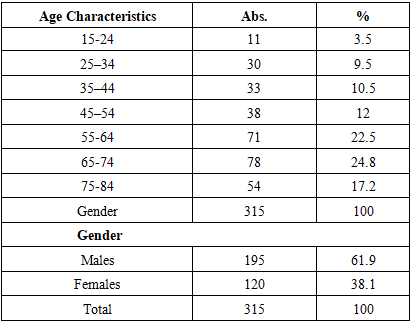
When studying any pathology, the age of the patients is of significant importance, as treatment measures depend on this factor. In this regard, we considered it appropriate to provide the age distribution of the examined patients, which is presented in the table below.Table 1. Age distribution of tuberculosis patients included in the study
 |
| |
|
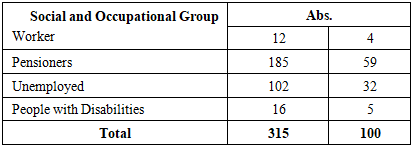
The obtained results indicate that the majority of affected individuals are elderly people over the age of 60, which supports the thesis that life expectancy is increasing and suggests successful treatment of this pathology. The low incidence among younger individuals (aged 19–40) reflects the high effectiveness of both specific and non-specific tuberculosis prevention measures within the population.In addition, we considered it appropriate to present the distribution of the examined patients according to their social status, the results of which are shown in Table 2.Table 2. Distribution of tuberculosis patients by social status
 |
| |
|
It is noteworthy that among those with tuberculosis, the number of pensioners, temporarily unemployed individuals, and housewives is relatively high. In the first case, these are age-based retirees, which indicates successful treatment of the disease and an increase in life expectancy among patients. In the second case, the disease creates difficulties for individuals in finding employment. In the third case, infection among housewives may be due to transmission of tuberculosis from family members. These findings highlight the reasons behind the high proportion of tuberculosis cases among these specific social groups.
3. Results and Discussion
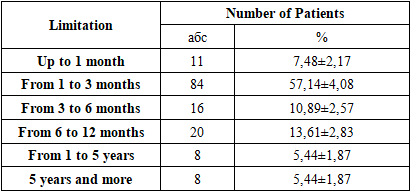
An important point in analyzing the obtained results is the nature of the patient's infection. When this pathology is first detected, it is defined as primary tuberculosis, whereas when the disease recurs, it is considered secondary tuberculosis. Secondary tuberculosis was identified after a long period of remission.Primary tuberculosis occurred 3.59 times more frequently than secondary tuberculosis, indicating that phthisiatricians primarily deal with patients suffering from the initial form of the disease. Due to the significant differences in infection and pathogenesis between the two forms, we used this classification in our study of the immunological aspects of tuberculosis.The isolation of the Mycobacterium tuberculosis pathogen by bacteriological methods also plays an important role in the progression of the disease. In addition, the localization of the pathological lesion is of great importance, as the location of the lesion determines the treatment strategy for the patient.It is well known that one of the main reasons for the low treatment efficacy in tuberculosis patients is the emergence of drug-resistant strains of the pathogen.Among the studied strains, one in four (n = 41) was drug-resistant (Mono-DR-TB, MDR-TB, XDR-TB, or PDR-TB), which significantly complicated the treatment of this pathology.Another important characteristic of the clinical material was the presence of comorbid conditions in the examined patients. Comorbidities were identified in 112 patients (76.19±3.51%), while 35 patients (23.81±3.51%) had no additional diseases.Notably, the most common comorbidities included cardiovascular diseases, diabetes mellitus, and viral hepatitis B and C, which can be attributed to the fact that most of the patients were elderly. Furthermore, each patient had an average of 2.27 nosological units, which further complicated the course of the primary disease.A specific feature of tuberculosis is that patients often report a range of subjective complaints upon seeking inpatient care. The frequency of these complaints among the examined patients is presented below.All 16 types of complaints reported were characteristic of tuberculosis, particularly pulmonary tuberculosis. On average, each patient reported 7.51 complaints, which underscores the importance of patient-reported symptoms as a key factor in confirming the diagnosis.In addition to clinical and laboratory studies, sputum samples were also examined. The results showed:53 positive cases (36.05±3.96%) by bacterioscopy,133 positive cases (90.48±2.42%) using the GeneXpert method,99 positive cases (67.35±3.87%) through DST (Drug Susceptibility Testing).These findings helped confirm the clinical diagnosis of tuberculosis and assess drug susceptibility of the pathogen.The duration of the disease is also a key factor in the adequate treatment of tuberculosis infection. Therefore, we present the duration of the disease among the examined patients in Table 3.Table 3. Comparative indicators of tuberculosis duration among the examined patients
 |
| |
|
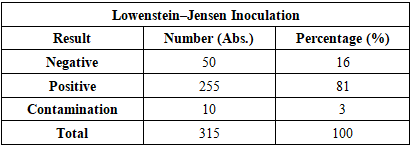
As shown in Table 3, the majority of patients sought medical care within the first 3 months—specifically, 7.48± 2.17% (n = 11) within the first month, and 57.14±4.08% (n = 84) within 1 to 3 months. This can be explained by the fact that most patients were diagnosed with primary tuberculosis.All diagnoses were confirmed in accordance with the International Classification of Diseases, 10th Revision (ICD-10). The obtained results are presented in the table.The main clinical form of pulmonary tuberculosis was infiltrative tuberculosis, observed in 77.55±3.44% (n = 114) of cases, which is consistent with findings reported by other researchers. Other forms were relatively rare (ranging from 1.36% to 4.76%). In addition to comorbid conditions, complications associated with the pathology were also identified in the examined patients. These are illustrated in Figure 2.The identified complications were found to worsen the course and prognosis of the disease. They were mainly associated with the respiratory and cardiovascular systems.Peripheral blood samples from patients at the Bukhara Regional Center of Phthisiology and Pulmonology (BotsFiP) were collected strictly in procedural rooms. Blood collection from tuberculosis patients was scheduled between 7:00 AM and 8:00 AM, and always on an empty stomach. According to standard procedure, 5 ml of blood was drawn from the cubital vein using single-use syringes.The whole blood was placed into a 10 ml conical glass centrifuge tube with graduation. This tube is cylindrical with a conical bottom, 17 mm in diameter, 110 mm in height, made of neutral glass, and marked with 0.2 ml divisions. The tube type is P-1-17-110 (GOST 1770-74). The maximum time from collection to delivery to the laboratory did not exceed 1.5 hours.The cellular composition of peripheral blood was analyzed, including the total leukocyte count, relative proportions of lymphocytes, monocytes, eosinophils, basophils, band and segmented neutrophils. Based on hematological tests, tables were compiled for statistical analysis of test results.Biochemical StudiesBiochemical blood analysis was performed using the Biossys 240 Plus automatic biochemical analyzer and the Sysmex CA-600 series ESR analyzer from Siemens.Leukocyte counts were carried out using the Zeiss Axiostar Plus optical binocular microscope.Bacteriological Research MethodsThe culture method is considered the gold standard for diagnosing tuberculosis and is mandatory for the interregional bacteriological laboratory of BotsFiP. To confirm the diagnosis of tuberculosis, it is necessary to isolate and identify Mycobacterium tuberculosis in artificial nutrient media.In routine studies, the standard medium for isolating the tuberculosis pathogen is the dense Lowenstein–Jensen nutrient medium. All procedures involving infectious material were carried out in strict compliance with technical and biological safety regulations.Tubes inoculated with culture were placed horizontally in a thermostat at 37°C for 24 hours so that the suspected mycobacteria could spread over the entire surface of the slanted agar, ensuring optimal growth. After 24 hours, the tubes were positioned vertically and incubated at the same temperature for up to 56 days. If no visible colony growth was observed after 56 days, the result was reported as negative.Sputum Examination MethodsFreshly produced sputum (10–15 ml) was collected in narrow-neck containers, and 0.5% NaOH solution was added. The resulting mixture was vigorously shaken for approximately 15 minutes. To further liquefy the sputum, 1 ml of xylene and 10 ml of distilled water were added, followed by another 15-minute shaking. Then, more distilled water was added until the liquid level reached the neck of the container, and the mixture was left to stand for 60 minutes.After one hour, a white layer appeared on the surface of the container. This layer was removed using a pipette and applied to sterile glass slides for further microscopic examination.Table 4. Inoculation onto Lowenstein–Jensen Medium
 |
| |
|
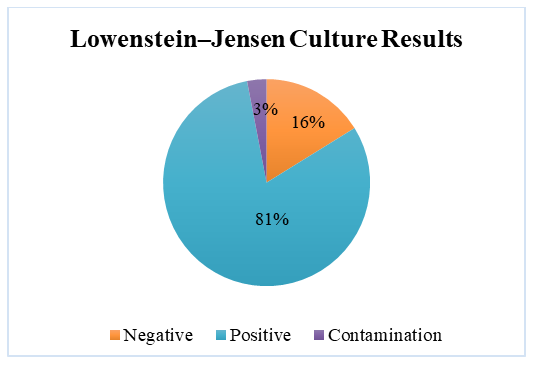 | Figure 1. Inoculation on Lowenstein–Jensen Medium |
 | Figure 2. Stages of inoculation on Lowenstein–Jensen medium |
Bacterioscopic MethodAll manipulations involving biomaterial in the bacterioscopic method were carried out in the BSL-3 zone. The studies were conducted inside a Labogene Scanlaf Mars Pro (Denmark) biological laminar flow cabinet. A smear was prepared by transferring biomaterial obtained from patients from a PP test tube to the center of a 2×1 cm glass slide using a disposable plastic bacteriological loop. The combined Ziehl–Neelsen staining method was used. The Ziehl–Neelsen staining included the following steps:First, Ziehl’s fuchsin was applied to the fixed specimen; to improve staining, a filter paper was placed on the slide. Using forceps to hold the slide, it was heated near the flame of a burner until vapors appeared. Fresh portions of the dye were added, and the heating was repeated twice more. After completing this staining, the slide was cooled, the filter paper removed, and rinsed with distilled water.The specimen was decolorized with a 5% Na₂SO₄ solution poured into the glass, then rinsed multiple times with water.The preparation was stained for 3–5 minutes with an aqueous-alcoholic solution of methylene blue, rinsed again with water, and dried. Finally, the slide was examined under an optical microscope.If rod-shaped bacteria stained red were observed in the field of view, the result was considered positive; the presence of contaminant microorganisms was indicated by blue staining.GeneXpert MethodThe Xpert MTB/RIF Ultra method for molecular genetic diagnosis of tuberculosis is currently considered the most accurate and advanced technique for diagnosing this pathology. Strict adherence to the specific workflow sequence is critical with this method. During operation, the GeneXpert device was connected to a computer equipped with the GeneXpert Dx System software.A prepared cartridge containing sputum from the patient requiring diagnosis was scanned using the barcode scanner, after which the test window appeared on the computer screen.Using barcode data, the software automatically populated fields such as test type, reagent lot number, cartridge serial number, expiration date, and others. Additional fields could be filled in if requested, e.g., the sample type (sputum or sputum sediment). Next, by pressing the "Start Test" button on the monitor screen, the operator waited for the module indicator to flash green and then inserted the cartridge. After closing the module door with the cartridge inside, the green light stopped flashing, indicating that the test had started. At the end of the test, the indicator light switched off. The cartridge was then removed and disposed of in a biohazard waste bag.All manipulations were performed wearing gloves. The following steps were carried out when introducing the biomaterial into the cartridge:The container lid was opened, and a sample of the biomaterial was added together with the sample reagent in a 1:2 ratio (e.g., 2 ml sample + 4 ml reagent). The lid was closed, and the container vortexed for 20–30 seconds, then left on the workbench for 10 minutes. The sample was vortexed again and left for another 5 minutes. After this procedure, the cartridge was opened, and 2 ml of the processed sample was transferred into the open port of the cartridge using a disposable pipette. The cartridge lid was then closed until a characteristic click was heard.If the result was MTB + RIF S (Mycobacterium tuberculosis detected, no rifampicin resistance), the result was displayed in black text on a green background; if MTB + RIF R (Mycobacterium tuberculosis detected, rifampicin resistance detected), it was displayed in black text on a red background. In the event of a test error, a message appeared in black text on a yellow background. If no result was detected or the result was invalid, a message appeared in black text on a gray background. Results were recorded in the laboratory journal, the Access electronic database, and the patient referral form.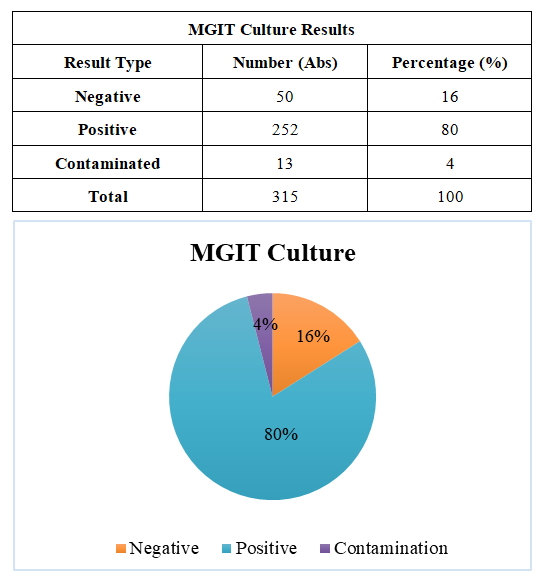 | Figure 3. MGIT Culture |
4. Conclusions
To perform DST (Drug Susceptibility Testing) using the proportional method, only pure cultures of mycobacteria are used, which have undergone a preliminary procedure. This includes checking for contamination with foreign microflora on blood agar and confirming the presence of Mycobacterium tuberculosis (MTB) by Ziehl–Neelsen staining of culture smears.The drug susceptibility of mycobacteria can be determined using either cultures grown in liquid medium or colonies cultivated on solid nutrient media. The study is carried out in accordance with the guidelines of an automated system. Currently, diagnostic kits are being developed for automated systems, allowing the determination of susceptibility of Mycobacterium tuberculosis to five main anti-tuberculosis drugs at the following concentrations (μg/ml): Levofloxacin (Lfx) – 1.0; Isoniazid (H) – 0.1; Rifampicin (R) – 1.0; Ethambutol (E) – 5.0; Pyrazinamide (PZA) – 100.0.
References
| [1] | Tosheva D. R. The prevalence of tuberculosis with extensively drug-resistant strains of the pathogen // Infection, Immunity and Pharmacology. 2024, No. 5. pp. 165–171. |
| [2] | Tosheva D.R. Prevalence of extensively drug-resistant tuberculosis // Journal of Humanities and Natural Sciences. 2024, No. 12. pp. 117–121. |
| [3] | Tosheva D.R. Changes in immunological parameters in various forms of the inflammatory process in tuberculosis // New Day in Medicine. 2024, No. 11 (73). pp. 148–152. |
| [4] | Tosheva D.R. Treatment of drug-resistant spinal tuberculosis using a combination of surgical intervention and individualized chemotherapy // New Day in Medicine. 2023, No. 10 (60). pp. 369–372. |
| [5] | Tosheva D.R. Causal Relationship of Immuno-Microbiological Parameters in Tuberculosis With Multiple and Broad Drug Resistance // International Journal of Health Systems and Medical Sciences. Vol. 2, Issue 5, 2023. pp. 259–262. |
| [6] | Tosheva D.R. Features of Drug Resistance of the Causative Agent of Tuberculosis in Modern Conditions // Journal of Applied and Medical Sciences. 2023, No. 4. pp. 59–63. |
| [7] | Tosheva D.R. Specifics of Drug Resistance of the Tuberculosis Pathogen in Current Conditions // Journal of Applied and Medical Sciences. 2023, No. 2. pp. 52–58. |
| [8] | Tosheva D.R. Isoniazid-Resistant Mycobacterium Tuberculosis: Frequency of Detection, Resistance Spectra, and Genetic Determinants of Resistance // American Journal of Pediatric Medicine and Health Sciences. 2023, No. 8. pp. 47–51. |
| [9] | Tosheva D.R. Evaluation of the Effectiveness of Vaccination for the Prevention of Tuberculosis in Children // Journal of Applied and Medical Sciences. 2023, No. 10. pp. 114–119. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


