-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(9): 3027-3030
doi:10.5923/j.ajmms.20251509.39
Received: Aug. 6, 2025; Accepted: Aug. 29, 2025; Published: Sep. 25, 2025

Use of a New Infusion Preparation Containing a Complex Compound of a Polysaccharide and a Natural Metabolite of the Krebs Cycle in Experimental Toxic Hypoxia
Larisa Shevchenko1, Jamoliddin Khujakhmedov2, Khamid Karimov3
1Blood Substitute Laboratory, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan
2Clinic Department, Viamed Clinic, Tashkent, Uzbekistan
3Department of Molecular Medicine and Cellular Technologies, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan
Correspondence to: Larisa Shevchenko, Blood Substitute Laboratory, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of this work is to evaluate the efficacy of Rheoambrasol on the expression of hypoxia markers (HIF-1α, erythropoietin), the activity of lipid peroxidation and antioxidant protection during single-step and prolonged nitrite intoxication. Experiments were carried out on male Wistar rats (n=185) using sodium nitrite on two models, i.e. on the model of acute nitrite hypoxia (NH). Experimental model of toxic methemoglobinemia. The results showed that single and prolonged use of sodium nitrite leads to methemoglobinemia, increased levels of hypoxic markers HIF-1α and erythropoietin (EPO), as a protective response to oxygen deficiency, activated lipid peroxidation and reduced overall antioxidant status. The new drug Rheoambrasol has an antihypoxic effect, which is confirmed by a decrease in values of hypoxia markers: HIF-1α and EPO, both in toxic hypoxia and in methemoglobinemia. Also, the corrective effect of the drug Rheoambrasol on the activity of lipid peroxidation and the activity of antioxidant protection enzymes under conditions of single and prolonged nitrite intoxication was established.
Keywords: Acute hypoxia, Methemoglobinemia, Polysaccharide complex, Natural metabolite, Hypoxia markers, Antioxidant status
Cite this paper: Larisa Shevchenko, Jamoliddin Khujakhmedov, Khamid Karimov, Use of a New Infusion Preparation Containing a Complex Compound of a Polysaccharide and a Natural Metabolite of the Krebs Cycle in Experimental Toxic Hypoxia, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 3027-3030. doi: 10.5923/j.ajmms.20251509.39.
Article Outline
1. Introduction
- As is known, among the means of pharmacological correction of pathological conditions accompanying intoxication, the key place in the treatment are drugs of antihypoxic and antioxidant action, capable at the cell level to restore metabolism and its vitality.The widespread distribution of xenobiotics in the modern world, which includes sodium nitrite, can lead to the development of hypoxia and formation of methemoglobin. Toxic hypoxia is accompanied by acute or chronic oxygen deficiency, impaired metabolism, cell functions, and can long have a significant impact on the formation and development of the underlying pathological process and increase the recovery time. At the same time hypoxia leads to energy deficit, activation of free radical processes in cells and decrease in reserves of mobilization of antioxidant protection [1,5,8].Considering the above, for pharmacological correction of toxic hypoxia we used a new blood substitute "Rheoambrasol", which has antihypoxic, antioxidant and membranoprotective effects, containing a complex compound of polysaccharide and natural metabolite of Krebs cycle [6].The aim of the work is to evaluate the effectiveness of “Rheoambrasol” on the severity of hypoxia markers (HIF-1α, erythropoietin), on the activity of lipid peroxidation, on antioxidant protection in experimental single-step and prolonged nitrite intoxication.
2. Main Body
2.1. Material and Methods of Study
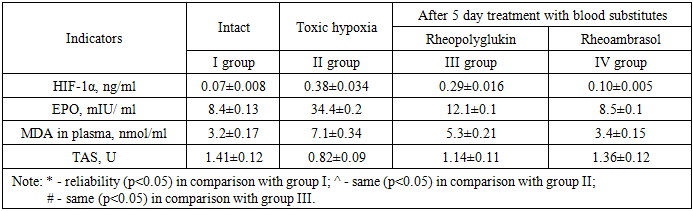
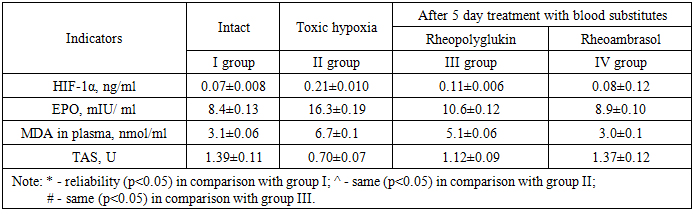
- The experiments were performed on 185 male rats weighing 190-220 g of the "Wistar" line in two models using sodium nitrite [3]. In the 1st series of experiments (on 100 rats) the model of acute nitrite hypoxia was reproduced by a single injection of 4% sodium nitrite solution under the back skin of rats at a dose of 90 mg/kg.Series 2 - model of toxic methemoglobinemia was created by prolonged intoxication, daily administration of sodium nitrite for 30 days at a dose of 50 mg/kg.All animals in series 1 used in the experiment were divided into the following IV groups: I - intact group consisted of rats on the normal laboratory diet, II - control group - animals with nitrite hypoxia without treatment, 48 hours after administration of the toxicant; III - comparison group - rats with nitrite hypoxia after infusion of “Rheopolyglukin”, IV - experimental group - rats with nitrite hypoxia after infusion of “Rheoambrasol”.injecting blood substitutes into the tail vein of rats at a dose of 5 ml/kg body weight for 5 days, 48 hours after sodium nitrite solution under administration in series 1, and 30 days after sodium nitrite solution under administration in series 2.The content of methemoglobin (metHb) in the blood of experimental animals was investigated in group II after sodium nitrite solution under injection in 1.5 hours and 48 hours in series 1, in series 2 in 1.5 hours and after the last sodium nitrite solution under injection on day 30, and in groups III and IV after treatment, in 1 hour and 5 days after administration of the studied preparations [2].The content of hypoxia-inducible factor (HIF-1α) was determined in the blood plasma of the experimental animals. The concentration of HIF-1α was determined by enzyme-linked immunosorbent assay (ELISA) using an enzyme immunoassay kit (Cloud-Clone corp., USA) according to the instructions enclosed with the kit.EPO concentration was determined by enzyme immunoassay using “Erythropoietin ELISA-BEST” kit (Vector-Best, Russia).Total antioxidant status (AOS) was also determined by enzyme immunoassay using an appropriate kit (Cayman, USA).Immunoassay results were measured at 450 and 630 nm on a microplate photometer MR96 (Mindray, China). The results were expressed in ng/ml.The intensity of lipid peroxidation (LPO) in erythrocyte hemolysates was determined by the level of malonic dialdehyde (MDA) according to the method of Titeeva G.R. (1996) [4]. Measurements were performed on a UNICO 2800 spectrophotometer (United products and instruments, Inc., USA).Statistical processing of the obtained data was performed using "Excel" and "Biostat 4.03" programs. The criterion for statistical significance was p<0.05.
2.2. Results of the Study
- The results of the study of methemoglobin showed that in the first series of experiments, the concentration of methemoglobin (metHb), under conditions of toxic hypoxia, already in 1.5 hours after the introduction of sodium nitrite increases by 56.6%. Then the concentration of metHb gradually decreases and by 48 hours we observe a partial recovery of the metHb concentration in the blood, but its value at the beginning of treatment is 13.2%. As seen in Figure 1, in the 1st series of experiments 48 hours after the first infusion of Rheoambrasol methemoglobin was restored to the baseline values, which was not observed after the use of Rheopolyglukin.
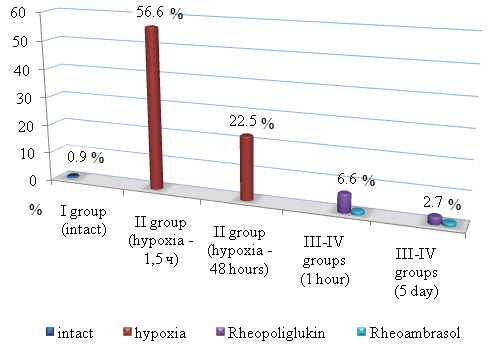 | Figure 1. Changes in methemoglobin concentration during hypoxia (series 1: after 1.5 hours and after 48 hours) and after treatment with blood substitutes (on the 1st and 5th days) |
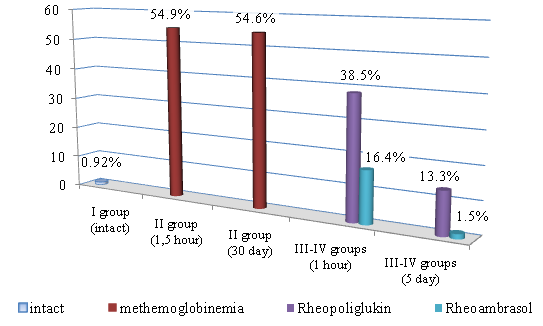 | Figure 2. Changes in methemoglobin concentration in the model of toxic methemoglobinemia (series 2: after 1.5 hours and at day 30) and after treatment with blood substitutes (at day 1 and day 5) |
|
|
3. Conclusions
- 1. With single and prolonged use of sodium nitrite methemoglobinemia develops, indices of hypoxic markers HIF-1α and EPO increase as a protective reaction to oxygen deficiency, lipid peroxidation processes are activated and general antioxidant status decreases.2. The antihypoxic effect of “Rheoambrasol” is confirmed by a decrease in the indices of hypoxia markers HIF-1α and EPO in both toxic hypoxia and methemoglobinemia.3. Corrective effect of “Rheoambrasol” on the activity of lipid peroxidation and the activity of antioxidant protection enzymes under conditions of single and prolonged nitrite intoxication was established.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML