-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(9): 2979-2982
doi:10.5923/j.ajmms.20251509.29
Received: Aug. 20, 2025; Accepted: Sep. 15, 2025; Published: Sep. 20, 2025

Comparative Profile of Antibiotic Susceptibility in E. coli and Salmonella spp. Strains Isolated from Patients with Acute Intestinal Infections and Broiler Chickens in the Republic of Uzbekistan
Tashpulatova M. K.1, Abdukhalilova G. K.1, Tashmuhamedova Sh. S.2, Khodzhaev M. M.2
1Republican Specialized Scientific and Practical Medical Center for Epidemiology, Microbiology, Infectious and Parasitic Diseases, Tashkent, Uzbekistan
2National University of Uzbekistan named after Mirzo Ulugbek, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

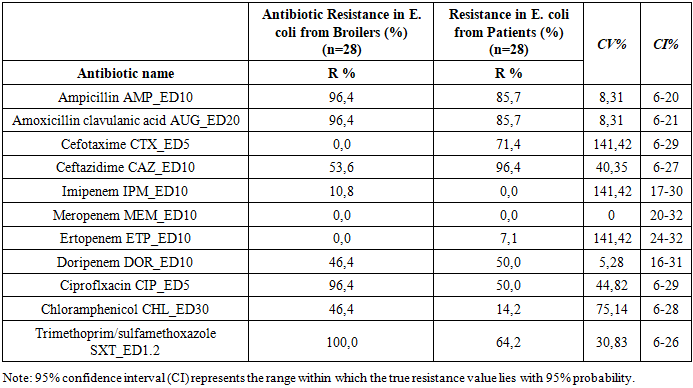
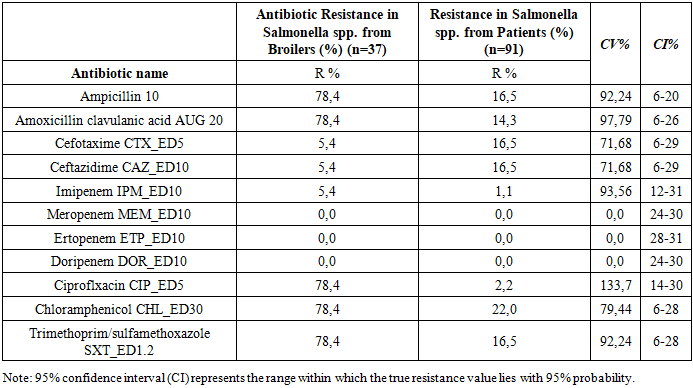
To monitor the antimicrobial resistance of Salmonella spp. and E. coli in the context of food safety, it is essential to assess their sensitivity to antimicrobial drugs (AMD) in order to adjust ongoing therapy. This aligns with the “One Health” initiative. Objective: To investigate the comparative antibiotic susceptibility of E. coli and Salmonella spp. strains isolated from patients with acute intestinal infections and from broiler chickens. Materials and Methods: A total of 56 E. coli and 128 Salmonella strains were studied using bacteriological methods at the Reference Laboratory of the Center for Antimicrobial Resistance (CAMR) between 2020 and 2021. Results: A high degree of resistance was observed in antibiotics such as Cefotaxime CTX_ED5, Imipenem IPM_ED10, and Ertapenem ETP_ED10 (CV% 141.42) in E. coli strains from broiler chickens compared to those from patients. Similarly, Salmonella spp. strains from both sources showed significant resistance to Ampicillin AMP_ED10, Amoxicillin-clavulanic acid AUG_ED20, Imipenem IPM_ED10, and Ciprofloxacin CIP_ED5 with CV% values of 92.24%, 97.79%, 93.56%, and 133.7%, respectively. This indicates widespread veterinary use of these drugs. Conclusion: Salmonella spp. strains from broilers exhibit higher levels of multidrug resistance than those from patients. E. coli strains from both sources show significant resistance to multiple antibiotic classes.
Keywords: Escherichia coli, Salmonell
Cite this paper: Tashpulatova M. K., Abdukhalilova G. K., Tashmuhamedova Sh. S., Khodzhaev M. M., Comparative Profile of Antibiotic Susceptibility in E. coli and Salmonella spp. Strains Isolated from Patients with Acute Intestinal Infections and Broiler Chickens in the Republic of Uzbekistan, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 2979-2982. doi: 10.5923/j.ajmms.20251509.29.
1. Introduction
- Salmonella and pathogenic Escherichia coli are among the most common foodborne pathogens, affecting millions of people each year, sometimes resulting in severe and even fatal outcomes. Foods most frequently associated with salmonellosis outbreaks include eggs, poultry, and other animal-derived products. Pathogenic E. coli is often linked to unpasteurized milk, undercooked meat, and contaminated fresh fruits and vegetables [7].Antimicrobial resistance (AMR) is one of the major global threats to public health and development. In 2019, bacterial AMR was directly responsible for 1.27 million deaths globally and contributed to an additional 4.95 million deaths [3]. The emergence of resistance is a natural biological response to antimicrobial use, which creates selective pressure that facilitates the selection, survival, and proliferation of resistant strains of microorganisms [1].E. coli is considered a multidrug-resistant bacterium due to its high potential for resistance to numerous antibiotics through various mechanisms. This includes the production of β-lactamase enzymes, which confer resistance to β-lactam antibiotics, as well as enzymes that induce resistance to aminoglycosides, quinolones, and others [8]. For instance, in countries reporting to the Global Antimicrobial Resistance Surveillance System (GLASS), the prevalence of resistance to ciprofloxacin ranges from 8.4% to 92.9% for Escherichia coli and from 4.1% to 79.4% for Klebsiella pneumoniae [9].Salmonella spp. ranks second only to Campylobacter among zoonotic pathogens responsible for human and animal infections. In the European Union (EU), approximately 100,000 cases of salmonellosis are reported annually [5]. Salmonella is a leading cause of foodborne outbreaks, often on an international scale. In 2018, about 35% of etiologically confirmed outbreaks (over 1,500 cases) in the EU were attributed to Salmonella, accounting for the largest numbers of affected, hospitalized, and deceased individuals [4].ObjectiveIt is essential to determine the antimicrobial susceptibility of Salmonella spp. and E. coli strains to optimize therapy and monitor resistance as part of food safety efforts under the “One Health” initiative [10]. This concept aims to ensure optimal health outcomes for humans, animals, and the environment by promoting sustainable balance among them. It emphasizes the interconnection between these domains to develop novel surveillance and disease control methods. The "One Health" approach covers issues such as antimicrobial resistance, foodborne illnesses caused by unsafe food, and zoonotic diseases—infectious diseases caused by microorganisms transmitted between animals and humans.
2. Materials and Methods
- To achieve the stated objective, studies were conducted at the “Center for Antimicrobial Resistance (CAMR)” laboratory of the Republican Specialized Scientific and Practical Medical Center for Epidemiology, Microbiology, Infectious and Parasitic Diseases (RSSPMCEMIPD), within the framework of the state grant project ID: PZ 20170928351, titled:"Development of a System for Forecasting and Preventing the Adverse Effects of Alimentary Factors on Human Health Based on Phenotypic Resistance and Common Antimicrobial Sensitivity Profiles in Microorganisms Isolated from Patients with Diarrhea and Agricultural Animals" (carried out from 03.01.2018 to 31.12.2020).Morphological, tinctorial, and biochemical properties of Salmonella spp. and E. coli strains were studied in accordance with WHO protocols and ISO 6579 standards [2,7].Biochemical activity was assessed by inoculating cultures into semi-liquid media containing various carbohydrates, alcohols, and amino acids: glucose, mannitol, dulcitol, urea, arabinose, xylose, citrate, acetate, malonate, phenylalanine, and lysine. Indole formation was determined using Morrel’s reagent in broth cultures.Antimicrobial susceptibility was assessed using the disk diffusion method according to EUCAST guidelines for 2020–2021 (versions 10.0–11.0). The results were interpreted based on the same EUCAST recommendations [6]. Mueller-Hinton agar (GEETA PHARMA, India) and antibiotic disks (Liofilchem, Italy) were used in the tests. Disk diffusion was the method applied to evaluate susceptibility. Salmonella and E. coli were tested for susceptibility to the following antimicrobial drugs: β-lactams: ampicillin, amoxicillin/clavulanic acid, cefotaxime, ceftazidime, imipenem, meropenem, ertapenem, doripenem, quinolones: ciprofloxacin, other classes: chloramphenicol, trimethoprim-sulfamethoxazole. For internal quality control, the reference strain E. coli ATCC 25922 was used.
3. Results and Discussion
- A total of 28 E. coli and 37 Salmonella spp. strains were isolated from broiler chicken carcasses (samples were collected from a poultry farm during slaughter), and 28 E. coli and 91 Salmonella strains were isolated from patients with acute intestinal infections (AII) hospitalized at RSSPMCEMIPD.To assess differences in resistance frequencies between E. coli and Salmonella spp. strains from broiler chickens and those from patients with AII, statistical analysis was performed. Strains with moderate or high resistance were grouped under the category “non-susceptible.”Tables 1 and 2 present the zones of growth inhibition for the microorganisms against various antimicrobial agents, along with 95% confidence intervals (CI) and coefficients of variation (CV%) to compare isolates from different sources (E. coli from broilers vs. patients; Salmonella spp. from broilers vs. patients).
|
|
4. Conclusions
- The absence of regulation and widespread use of antibiotics in clinical practice leads to the development of bacterial resistance to antimicrobial agents. Bacteria employ a variety of resistance mechanisms to protect themselves. Acquired resistance arises from mutations, gene transfer through conjugation or transformation, transposons, and bacteriophages. It is essential to determine bacterial resistance across all classes of antibiotics (phenotypic analysis), as well as identify genetic mutations responsible for resistance (genotypic analysis). A deeper understanding of the mechanisms of antibiotic action, the chromosomal locations of resistance genes, and their expression would enable the development of screening and control strategies necessary to reduce the spread and evolution of resistant bacteria.A statistically significant level of resistance was observed for antibiotics such as Cefotaxime CTX_ED5, Imipenem IPM_ED10, and Ertapenem ETP_ED10 with a CV% of 141.42 when comparing E. coli strains from broiler chickens and those from patients with acute intestinal infections. The frequency of resistant Salmonella spp. strains from different sources also demonstrated significant resistance to Ampicillin 10, Amoxicillin/clavulanic acid AUG 20, Imipenem IPM_ED10, and Ciprofloxacin CIP_ED5, with CV% values of 92.24%, 97.79%, 93.56%, and 133.7%, respectively. This indicates high statistical reliability and reflects the extensive use of these antibiotics in veterinary medicine. Thus, Salmonella spp. strains isolated from broiler chickens exhibit higher levels of multidrug resistance than strains isolated from patients with acute intestinal infections. E. coli strains, whether isolated from broilers or patients, show high resistance to multiple classes of antibiotics.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML