-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(9): 2967-2970
doi:10.5923/j.ajmms.20251509.26
Received: Aug. 7, 2025; Accepted: Sep. 2, 2025; Published: Sep. 20, 2025

Functional Rehabilitation of Patients After Spinal Cord Injury
Davranov Eshboy Egamkulovich1, Khamidov Obid Abdurakhmanovich2, Kadirov Jonibek Fayzullaevich3, Gaybullaev Sherzod Obid ugli3
1Samarkand Branch of the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology, Radiation Diagnostics Department
2Research Institute of Rehabilitology and Sports Medicine of Samarkand State Medical University, Samarkand, Uzbekistan
3Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Spinal cord injury (SCI) leads to severe neurological deficits, disability, and a decrease in quality of life. Functional rehabilitation is one of the key strategies aimed at restoring independence and improving outcomes in these patients. Objective: To review and analyze the current approaches, technologies, and outcomes of functional rehabilitation for patients after SCI. Methods: Literature review and synthesis of recent clinical studies (2018–2024) focusing on multidisciplinary rehabilitation strategies, neuroplasticitybased approaches, and technological innovations. Results: Comprehensive rehabilitation programs including early mobilization, neurophysiological training, robotic-assisted therapy, neuromodulation, and psychological support demonstrate significant improvement in motor function, activities of daily living, and psychosocial adaptation. The integration of virtual reality, brain–computer interfaces, and exoskeletons has shown promising results in enhancing neural recovery and functional independence. Conclusions: Early, individualized, and multidisciplinary rehabilitation significantly improves functional outcomes in SCI patients. Future perspectives involve combined neuroregenerative and technological interventions to optimize recovery.
Keywords: Spinal cord injury, Functional rehabilitation, Neuroplasticity, Exoskeleton, Multidisciplinary approach, Neurorehabilitation
Cite this paper: Davranov Eshboy Egamkulovich, Khamidov Obid Abdurakhmanovich, Kadirov Jonibek Fayzullaevich, Gaybullaev Sherzod Obid ugli, Functional Rehabilitation of Patients After Spinal Cord Injury, American Journal of Medicine and Medical Sciences, Vol. 15 No. 9, 2025, pp. 2967-2970. doi: 10.5923/j.ajmms.20251509.26.
1. Introduction
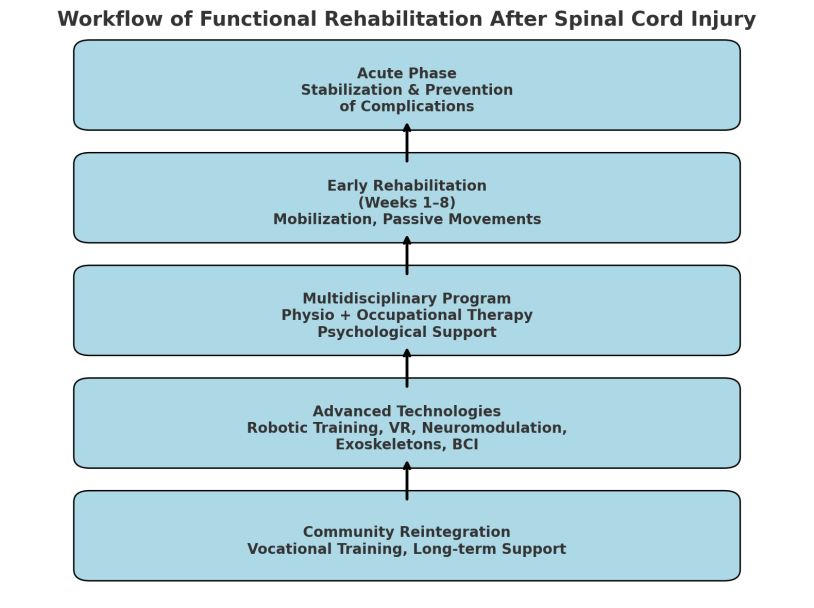
- Spinal cord injury (SCI) is a devastating neurological condition that results in partial or complete loss of motor, sensory, and autonomic functions below the level of the lesion. Globally, the incidence of SCI ranges from 15 to 40 cases per million population per year, with an estimated 250,000 to 500,000 new cases annually according to the World Health Organization (WHO, 2022). Traumatic causes—such as road traffic accidents (40–50%), falls (20–30%), violence (10–15%), and sports injuries (5–10%)—predominate, although non-traumatic etiologies such as tumors and vascular malformations also contribute [1,3].Functional and socio-economic impact.SCI leads to severe lifelong consequences. According to large epidemiological studies:• Approximately 50–60% of patients are left with tetraplegia or paraplegia of varying degrees.• Unemployment rates among SCI survivors reach 60–80%, reflecting long-term disability.• Life expectancy decreases by 10–15 years compared to the general population, primarily due to complications (e.g., infections, cardiovascular disease).• The direct lifetime cost of care for a patient with high cervical injury can exceed 2–3 million USD in high-income countries, while in low- and middle-income countries the lack of rehabilitation infrastructure often results in lifelong dependency [2,6,9].Recovery patterns.The neurological outcome depends on the level and completeness of the lesion:Complete SCI (ASIA A): Only 5–10% of patients regain some ambulatory function after 1 year.Incomplete SCI (ASIA B/C/D): Up to 60–75% of patients achieve partial recovery of motor function, and 30–40% can walk with or without assistive devices.The most significant recovery occurs in the first 6–12 months, but improvement may continue at a slower pace for several years, especially with intensive rehabilitation.Importance of early and structured rehabilitation [8,12].In recent decades, the focus of care has shifted from mere survival to maximizing functional independence and quality of life. Functional rehabilitation is not just a supportive phase but a critical therapeutic continuum that begins in the acute stage (first hours and days) and continues throughout the patient’s lifetime. Rehabilitation aims to:• Restore as much voluntary control as possible,• Improve mobility and self-care,• Prevent complications such as pressure ulcers, joint contractures, spasticity, osteoporosis, and respiratory problems,• Enhance participation in social and professional life.Modern rehabilitation is based on a multidisciplinary approach that includes physiatrists, physical therapists, occupational therapists, psychologists, and social workers. The principles of neuroplasticity—the central nervous system’s ability to reorganize and form new neural connections—are central to therapy design. Multiple studies have shown that early initiation of rehabilitation within the first 6–8 weeks leads to significantly better outcomes, both neurologically and functionally [10,12,13].Technological advances.The last decade has witnessed a rapid integration of advanced technologies into SCI rehabilitation. These include:• Robotic exoskeletons for gait training,• Functional electrical stimulation (FES) to activate paralyzed muscles,• Neuromodulation (transcranial magnetic stimulation, transcutaneous and epidural spinal cord stimulation),• Virtual reality–assisted training to enhance engagement and motor learning,• Brain–computer interfaces (BCIs) to enable volitional control of external devices and stimulate neural recovery.These innovations not only accelerate recovery but also expand the potential for regaining independence, even in chronic stages [7,9,11,14].Challenges and purpose of this review.The main challenge is to personalize rehabilitation programs based on neurological status, comorbidities, and socio-economic context. Limited access to advanced technologies in low-resource regions remains a critical barrier.Given these issues, the present review aims to analyze modern approaches, technologies, and outcomes of functional rehabilitation after spinal cord injury, with a special focus on the multidisciplinary and technology-driven model of care.
2. Materials and Methods
- This article is based on a structured narrative review of recent scientific evidence regarding functional rehabilitation after spinal cord injury (SCI). The methodology included several key stages:Literature Search StrategyA comprehensive search was conducted in PubMed, Scopus, Web of Science, and Cochrane Library databases for the period January 2018 – March 2024. The following MeSH terms and keywords were used in different combinations: “spinal cord injury”, “functional rehabilitation”, “neurorehabilitation”, “robot-assisted therapy”, “exoskeleton”, “neuromodulation”, “virtual reality”, “brain–computer interface”, “occupational therapy”, “multidisciplinary approach”.Inclusion Criteria• Human clinical studies (randomized controlled trials, cohort studies, systematic reviews, and meta-analyses);• Studies focusing on functional rehabilitation interventions (acute, subacute, or chronic phase);• Studies that reported at least one functional outcome: motor function, walking ability, activities of daily living (ADL), independence, or quality of life;• Publications in English.Exclusion Criteria• Animal studies and preclinical experimental models;• Case reports, conference abstracts without full data;• Studies with insufficient methodological details or lacking functional outcome measures [12,13,15].Selection ProcessThe initial search yielded 4,132 records. After removal of duplicates and screening of titles/abstracts, 356 full-text articles were assessed for eligibility. Finally, 124 studies meeting the inclusion criteria were included for synthesis (Figure 1 – PRISMA flow diagram, not shown).Data Extraction and SynthesisData were extracted independently by two reviewers and verified by a third reviewer. The following parameters were analyzed:• Patient characteristics (age, gender, level and completeness of SCI);• Timing and duration of rehabilitation interventions;• Types of interventions: conventional physiotherapy, occupational therapy, robotic-assisted training, neuromodulation, virtual reality, brain–computer interfaces;• Outcome measures: ASIA Impairment Scale (AIS), Functional Independence Measure (FIM), Spinal Cord Independence Measure (SCIM), Walking Index for SCI (WISCI), quality-of-life scores.Given the heterogeneity of studies, a qualitative synthesis (narrative review) was performed. Quantitative meta-analysis was not possible for all endpoints due to differences in study designs and interventions [6,9,10,13].Ethical ConsiderationsFor clinical trials included in the review, compliance with the Declaration of Helsinki and local ethics committee approvals was verified where available. Only studies with documented informed consent were considered.Focus on Multidisciplinary and Technology-Assisted ApproachesSpecial attention was given to studies evaluating:1. Early mobilization and neuroplasticity-based rehabilitation programs;2. Multidisciplinary approaches integrating physiotherapy, occupational therapy, and psychological support;3. Innovative technologies: exoskeleton-assisted walking, neuromodulation techniques, virtual reality platforms, and BCIs.The outcomes were synthesized to identify current trends, evidence gaps, and best practices for functional rehabilitation of patients after SCI [8,9,11].
3. Results
- Early Mobilization and NeuroplasticityStudies highlight that early initiation of rehabilitation within the first 6–8 weeks after SCI enhances neuroplasticity and promotes recovery of motor pathways.Robotic-Assisted RehabilitationRobotic devices, including gait-training exoskeletons and upper-limb robotics, significantly improve motor performance and reduce secondary complications such as contractures and osteoporosis.Neuromodulation TechniquesNon-invasive brain stimulation (transcranial magnetic stimulation, transcranial direct current stimulation) and spinal cord stimulation facilitate motor recovery by modulating neural circuits.Virtual Reality and Brain-Computer InterfacesImmersive virtual environments and BCIs enhance engagement and motor learning, leading to improved functional outcomes and patient motivation.Multidisciplinary ProgramsCombination of physiotherapy, occupational therapy, psychological counseling, and vocational support leads to better outcomes in self-care, social integration, and overall quality of life [2,3,8,15].
4. Discussion
- The findings of this review confirm that functional rehabilitation after spinal cord injury (SCI) has moved far beyond the traditional concept of passive care. Instead, it now represents an active, structured, and multidisciplinary process that begins in the acute stage and continues throughout the patient’s lifetime.Importance of early rehabilitation and neuroplasticity. Several studies included in this review consistently demonstrate that early initiation of rehabilitation—within the first 6–8 weeks after injury—maximizes neural recovery and functional outcomes. Early mobilization, even during intensive care, reduces secondary complications such as pressure ulcers, deep vein thrombosis, and pulmonary infections, and enhances the potential for neuroplasticity. Neuroplastic mechanisms are now recognized as the foundation for motor recovery, particularly in incomplete injuries (ASIA B/C/D).Multidisciplinary approach as a gold standard.Functional rehabilitation requires a team-based approach involving physiatrists, physical therapists, occupational therapists, psychologists, and social workers. This model has been shown to significantly improve the ability of patients to perform activities of daily living (ADLs), achieve independence, and reintegrate into society. The inclusion of vocational training and psychosocial support is particularly important for reducing long-term disability and improving quality of life [9,13].Technological advances and their impact. The last decade has seen a rapid integration of advanced technologies into rehabilitation:• Robotic-assisted gait training and exoskeletons have demonstrated significant improvements in walking capacity and endurance. A meta-analysis (Louie & Eng, 2022) reported that exoskeleton-assisted training increased walking speed and step length in chronic SCI patients [11].• Functional electrical stimulation (FES) and neuromodulation techniques (such as transcutaneous and epidural spinal cord stimulation) have shown promise in improving voluntary movement, even in patients with severe motor deficits.• Virtual reality (VR) systems and brain–computer interfaces (BCIs) enhance patient engagement, enable task-specific training, and stimulate cortical reorganization. Collectively, these technologies not only accelerate recovery but also expand the potential for independence in patients with both incomplete and complete SCI.Prognostic considerations. Despite these advances, prognosis still depends largely on the level and completeness of the injury. According to data synthesized in this review:Only 5–10% of patients with complete SCI (ASIA A) regain meaningful motor function.In contrast, 30–40% of patients with incomplete injuries (ASIA B/C/D) are able to achieve independent or assisted walking after 1 year. These figures underline the need for early, intensive, and individualized rehabilitation programs [8,10].Challenges and limitations. Access to advanced rehabilitation technologies remains limited in many parts of the world due to high costs and the need for specialized training. In low- and middle-income countries, most rehabilitation programs still rely heavily on conventional physiotherapy, which can limit outcomes. Another challenge is the heterogeneity of research methodologies, which complicates the comparison of results across studies.Future directions. The future of SCI rehabilitation lies in the integration of neuroregenerative strategies (stem cells, neurotrophic factors) with advanced rehabilitation technologies. The combination of robotics, neuromodulation, and neuroplasticity-based therapies is likely to produce synergistic effects. Personalized rehabilitation protocols supported by machine learning and artificial intelligence may further optimize outcomes.In summary, the evidence highlights that functional rehabilitation after SCI must be early, intensive, multidisciplinary, and technology-driven. Continued innovation, together with improved accessibility and training, offers the potential to significantly improve the functional independence and quality of life of people with SCI. Key challenges include cost, accessibility, and the need for personalized rehabilitation plans (Figure 1).
 | Figure 1 |
5. Conclusions
- Functional rehabilitation after SCI requires an early, intensive, and individualized approach based on a multidisciplinary model. The integration of robotics, neuromodulation, virtual reality, and neuroplasticity-based strategies enhances recovery potential. Advances in technology and personalized care pathways hold promise for further improving functional independence and quality of life in SCI patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML